Abstract
Objective: This study evaluated IDDSI syringe-flow behaviour of paediatric formulas in Türkiye under laboratory conditions when prepared with a maltodextrin/carob‑gum thickener commonly used locally and is marketed for reflux. Findings concern IDDSI flow under laboratory conditions and do not constitute clinical recommendations.
Methods: Fifteen commercial paediatric formulas (5 powdered, 10 liquid) were prepared with the thickener at 1.7 g/100 mL using a 7-minute rest protocol. The IDDSI syringe flow test (10 mL, 10 s) was performed at 0, 7, 15, 30, and 45 minutes. Two independent researchers assessed flow; inter-rater reliability was evaluated. Preparation water was 37–38 °C; testing occurred under standard room conditions; samples were covered between timepoints. Linear regression examined potential associations between thickening success, formula type, and macronutrient content.
Results: At baseline, all formulas were classified as IDDSI Level 0. After 45 minutes, 8 of the 15 formulas (53%) reached IDDSI Level 1 or higher. A higher percentage of liquid formulas (60%) thickened successfully compared to powdered formulas (40%), although this difference was not statistically significant. The thickness level increased significantly over time (p < 0.001), but the regression model revealed no significant associations between thickening success and formula type or macronutrient composition (p > 0.05).
Conclusion: Under these standardised laboratory conditions, fewer than half of tested formulas reached IDDSI Level 1 with this maltodextrin/carob-gum thickener. Outcomes varied by product and were not predictable from nutritional content or formula type. Product-specific testing is warranted, and clinical studies are required before informing practice.
Keywords: IDDSI, infant formula, thickener, viscosity, formula consistency
Main Points
- Thickening is Inconsistent: Even when following a 7-minute rest protocol, a majority of formulas failed to reach the desired IDDSI Level 1 consistency, with only one of 15 formulas meeting the criteria at this time point.
- Nutritional Content Isn’t a Predictor: The study found no statistically significant association between a formula’s macronutrient content (e.g., protein, fat, or carbohydrates) and its ability to thicken.
- Standardized Protocols Are Needed: The study concludes that the lack of clear manufacturer instructions on the appropriate waiting time and amount of thickener for specific IDDSI outcomes makes the product’s use insufficient for safe paediatric care.
Introduction
Paediatric feeding disorders (PFDs), defined as the inability to consume an age-appropriate diet orally, are a common clinical diagnosis. The prevalence of PFDs is known to be higher in children with a history of premature birth, neuromuscular disorders, cardiopulmonary disorders, upper respiratory-digestive system anomalies, and gastrointestinal system disorders.1 A frequent underlying contributor to PFD is dysphagia-a swallowing disorder that compromises the safety, efficiency, or adequacy of food or fluid intake.2
Dysphagia affects approximately 590 million people (8%) worldwide, with a prevalence of around 0.9% in the paediatric population.3,4 Considering that approximately 25% of children with dysphagia have otherwise typical development, the problem is more widespread than is often assumed.1,4,5 Dysphagia is classically categorised as oropharyngeal or oesophageal, arising from structural or motility disorders.6 Oropharyngeal dysphagia (OD) refers to any abnormality in the physiology of swallowing within the upper gastrointestinal tract. This can include an imbalance in the coordination between respiratory and feeding functions, leading to clinical complications such as malnutrition, aspiration pneumonia, and premature death.7,8 OD can severely reduce quality of life by compromising nutrition and ventilation, as oropharyngeal secretions, food, or liquids may enter the airway.9
In children with dysphagia, limited oral intake often leads to unmet energy and macronutrient needs, elevating the risk of malnutrition.6,10,11 Therefore, feeding therapy is typically the first-line intervention, often requiring a multidisciplinary approach. Both enteral nutrition and therapeutic strategies aim to enhance nutritional status and improve overall quality of life in these children.
Texture-modified foods and thickened liquids are commonly used in dysphagia management to support safer intake without compromising nutritional adequacy.3,6,11Thickeners are widely used for this purpose, as they can slow bolus transit and improve cohesion during swallowing.12-14 Although a wide range of thickeners—food-based and commercial—are used in paediatric15, efforts to standardise terminology (e.g., the International Dysphagia Diet Standardisation Initiative, IDDSI) are challenged in practice by variability in preparation instructions and product-specific performance.16 These issues make product-level evaluation using the IDDSI syringe-flow framework clinically relevant.
Dysphagia and gastro-oesophageal reflux (GERD) are distinct clinical entities. The product evaluated here is marketed locally for reflux and is not approved for dysphagia; accordingly, this study examines laboratory IDDSI syringe-flow behaviour only and does not assess clinical outcomes or imply clinical efficacy. In Türkiye, a maltodextrin/carob-gum thickener is routinely used in paediatric feeding practice and, to our knowledge, is effectively the sole option in routine use; therefore, we adopted a single-thickener design to reflect real-world availability.
The primary objectives of this study were to:
- Determine whether commercially available paediatric formulas achieve IDDSI Level 1 (slightly thick) consistency when prepared with a commonly used starch-based thickener in Türkiye.
- Assess whether formula type and macronutrient composition influence thickening outcomes.
- Evaluate the time to reach target consistency and the stability of thickness over time.
Methods
This study evaluated the effectiveness of a widely used commercial paediatric thickener in Türkiye for achieving desired consistency in infant and child formulas. We also assessed the thickener’s preparation instructions due to the lack of standardized guidance on the amount and thickening time. A total of 15 formulas, including both powder-based (n=5) and ready-to-feed liquid (n=10) varieties commonly used in paediatric clinical settings, were included in the analysis.
Sample preparation
Each formula sample (100 mL) was prepared according to the manufacturer’s instructions. Bebelac Gold thickener (Numil Food Products Industry and Trade Inc., Türkiye), composed of maltodextrin and carob gum, was added at a concentration of 1.7 g per 100 mL. Preparation water was 37–38 °C; testing occurred under standard room conditions (21- 22°C ambient). The mixture was stirred horizontally for 5 seconds and vertically for 20 seconds, then allowed to rest for 7 minutes to approximate the viscosity stabilization time of starch-based thickeners.17-19
Flow test
The consistency of the thickened formulas was assessed using the International Dysphagia Diet Standardization Initiative (IDDSI) flow test, in accordance with the IDDSI Framework and Testing Methods.3,20 The test was conducted using a 10 mL slip-tip syringe, which was validated for compatibility with IDDSI standards in terms of barrel length and flow characteristics. Syringes were checked prior to testing to ensure standardization, as variations in dimensions can affect measurement accuracy.20
To perform the test, 10 mL of each sample was drawn into the syringe, and the nozzle was unblocked for 10 seconds. The IDDSI level was determined by measuring the volume remaining in the syringe after 10 seconds of flow time:
IDDSI Level 0 (Thin): <1 mL remaining
IDDSI Level 1 (Slightly Thick): 1-4 mL remaining
IDDSI Level 2 (Mildly Thick): 4-8 mL remaining
IDDSI Level 3 (Moderately Thick): 8-10 mL remaining
Flow tests were performed under consistent environmental and serving conditions, including temperature, to ensure clinical relevance.20 All measurements were conducted in duplicate. If the difference between the two measurements exceeded 1 mL, a third trial was conducted.
Measurements were taken at five post-preparation time points (baseline (without thickener), 7, 15, 30, and 45 minutes) to evaluate the stability of the thickened liquids over time. All tests were carried out independently by two researchers to ensure inter-rater reliability. Only samples initially identified as IDDSI Level 0 were subjected to thickening procedures. The primary outcome was whether the thickened formula achieved IDDSI Level 1 following the standardized thickening and resting protocols.
Statistical analyses
Data were analysed using R version 4.3.1 and SPSS version 26. Descriptive statistics were used to summarize IDDSI success rates and viscosity changes over time. Inter-rater reliability between assessors was evaluated using the intraclass correlation coefficient (ICC) with 95% confidence intervals.
Independent samples t-tests were conducted to compare the mean nutritional content (Energy, Protein, Fat, Saturated fat, Carbohydrates, Sugar, Fibre) between formulas classified as IDDSI Level 0 and those classified as IDDSI Level 1 or higher at 45 minutes.
An exploratory linear regression analysis was conducted to investigate potential associations between macronutrient content and the time required to reach IDDSI Level 1. Given the study sample size (n=15), these analyses are interpreted as preliminary observations rather than predictive modelling. Additionally, repeated measures ANOVA were conducted to assess changes in flow test results across the five time points (0, 7, 15, 30, and 45 minutes). For all statistical tests, a p-value < 0.05 was considered statistically significant.
Results
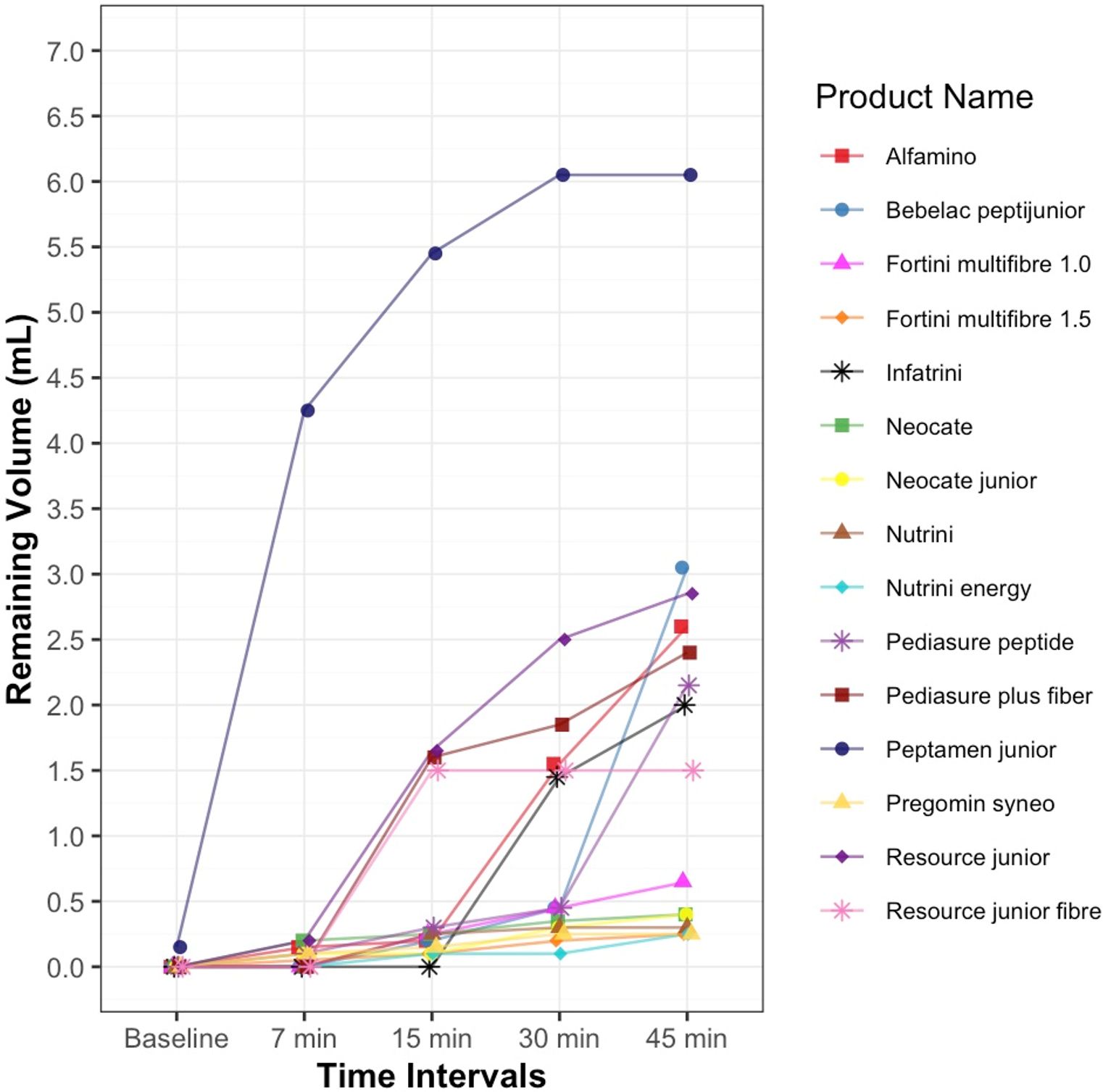
A total of fifteen infant and child formulas, including both powder-based and ready-to-feed liquid products, were included in the analysis. At baseline, all formulas (100%) were classified as thin (IDDSI level 0). Peptamen junior (liquid) consistently over-thickened to Level 2. Alfamino (powder) demonstrated stability, maintaining Level 1 after 15 minutes (Figure 1).
Table 1 summarizes the thickening performance and macronutrient composition of the 15 commercial formulas categorized as hypoallergenic, oral, and enteral. Among oral formulas, a greater proportion successfully thickened (e.g., Pediasure and Resource Junior variants). Although, Peptamen Junior, a high-protein formula, thickened beyond the target to Level 2. Notably, none of the enteral formulas achieved the desired consistency, highlighting variability across categories despite identical thickening protocol. On average, formulas contained 2.9 g of protein, 13.4 g of carbohydrates, and 5.1 g of fat per 100 mL (Table 1).
| Table 1. Thickening outcomes, and macronutrient composition of infant and child formulas by product type | |||
| Product Type |
|
|
|
| Hypoallergenic |
|
||
| Alfamino |
|
|
|
| Bebelac peptijunior |
|
|
|
| Neocate |
|
|
|
| Neocate junior |
|
|
|
| Pregomin syneo |
|
|
|
| Oral |
|
||
| Fortini multifibre 1.0 |
|
|
|
| Fortini multifibre 1.5 |
|
|
|
| Infatrini |
|
|
|
| Pediasure peptide |
|
|
|
| Pediasure plus fiber |
|
|
|
| Peptamen junior |
|
|
|
| Resource junior |
|
|
|
| Resource junior fibre |
|
|
|
| Enteral |
|
||
| Nutrini |
|
|
|
| Nutrini energy |
|
|
|
| Macronutrients |
|
|
|
| Energy (kcal/100mL) |
|
|
|
| Protein total (g/100 mL) |
|
|
|
| Carbohydrate total (g/100 mL) |
|
|
|
| Sugar (g/100 mL) |
|
|
|
| Fibre (g/100 mL) |
|
|
|
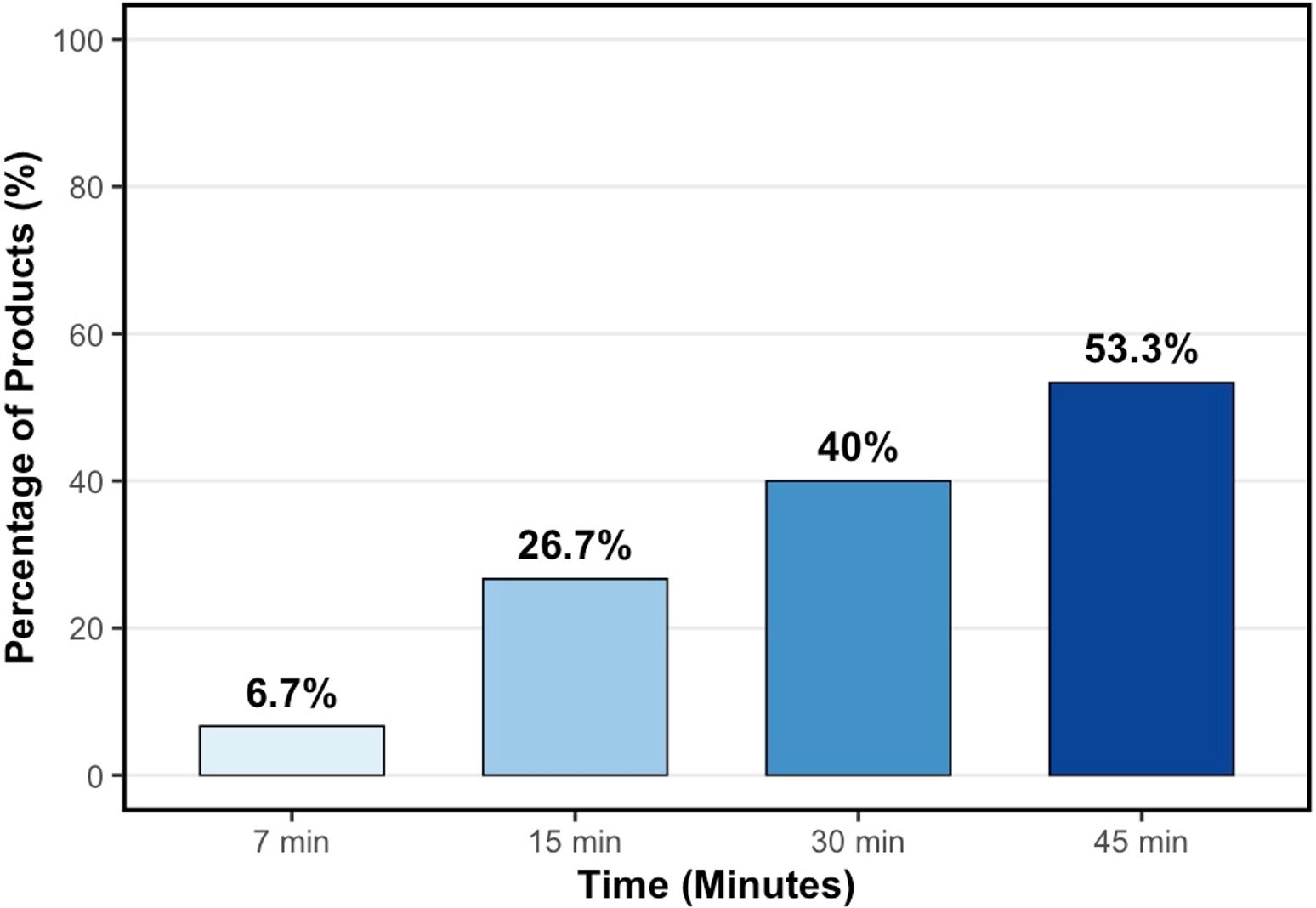
Thickening efficacy was assessed at 45 minutes, with 53% of all formulas achieving at least IDDSI Level 1 (Slightly Thick) (Figure 2).
A significant main effect of time was observed in the repeated measures ANOVA, F (1.81, 23.53) = 12.89, p < 0.001, indicating that thickness (measured by flow remaining) changed significantly over the 45-minute period. However, when comparing powder and liquid formulas at each time point, no statistically significant differences were found (p > 0.05). Descriptive trends in flow remaining by formula type are presented in Table 2.
|
Time: F (1.81, 23.53) = 12.89, p < 0.001 Formula Type: F (1, 13) = 0.834, p = 0.378 Time x Formula Type Interaction: F (1.81, 23.53) = 0.895, p = 0.413 SD: Standard Deviation, Greenhouse-Geisser correction applied for within-subject’s effects due to violation of sphericity. |
|||
| Table 2. Mean remaining volume (mL) over time by formula type | |||
| Time Point |
|
|
|
| 7 minutes |
|
|
|
| 15 minutes |
|
|
|
| 30 minutes |
|
|
|
| 45 minutes |
|
|
|
Across all time points, the difference between the two researchers’ measurements did not exceed 1 mL for any sample, thus a third trial was not required. Inter-rater reliability was consistently excellent, with intraclass correlation coefficients (ICC) ranging from 0.89 at baseline to 0.99 at all post-thickening time points. The 95% confidence intervals ranged from 0.67 to 1.00, confirming a high degree of measurement agreement between researchers using the IDDSI syringe method.
Regarding the nutritional content of formulas grouped by IDDSI level at 45 minutes, no statistically significant differences were found for Energy, Protein, Fat, Carbohydrates, Sugar, and Fibre between formulas achieving Level 0 and those achieving Level 1 or higher (p > 0.05 for all, Table 3).
| t-test, SD: Standard Deviation | |||||
| Table 3. Assessment of nutritional content of formulas by IDDSI level (45-minute flow test results) | |||||
|
|
|
|
|
|
|
| Energy |
|
|
|
|
|
|
|
|
|
|
||
| Protein |
|
|
|
|
|
|
|
|
|
|
||
| Fat |
|
|
|
|
|
|
|
|
|
|
||
| Saturated fat |
|
|
|
|
|
|
|
|
|
|
||
| Carbohydrates |
|
|
|
|
|
|
|
|
|
|
||
| Sugar |
|
|
|
|
|
|
|
|
|
|
||
| Fibre |
|
|
|
|
|
|
|
|
|
|
||
A linear regression model was conducted to evaluate whether formula type and macronutrient content could predict the time required to reach IDDSI Level 1 consistency. None of the predictors showed a statistically significant association with time-to-thickening (p > 0.05). The model explained 34% of the variance (R² = 0.343) (Table 4).
| ¹ Formula Type coded as 0 = Liquid, 1 = Powder. All macronutrient coefficients reflect change per gram per 100 mL of formula. The dependent variable is the minimum time (in minutes) at which each formula first reached IDDSI Level 1 or higher (i.e., Level 1, 2, or 3). Model R² = 0.343, Adjusted R² = 0.080, p= 0.332. | ||
| Table 4. Linear regression predicting time to IDDSI level 1 | ||
| Predictor |
|
|
| Intercept |
|
|
| Formula Type1 |
|
|
| Protein |
|
|
| Fat |
|
|
| Carbohydrates |
|
|
Discussion
This study evaluated the effectiveness of a starch-based thickener in achieving IDDSI Level 1 consistency in infant and child formulas commonly used in Türkiye. Despite following the manufacturer’s preparation and rest protocols, only 53% of formulas achieved the desired consistency after 45 minutes. Liquid formulas demonstrated a higher success rate than powder-based formulas (60% vs. 40%), but this difference was not statistically significant.
Compared to other literature, our results show lower thickening success rates. For example, Frakking et al.19 found that approximately 75% of Australian formulas reached IDDSI Level 1 using similar protocols. Differences in thickener type, formula composition, and testing time likely explain this variability. Our results are more in line with Ng et al.21, who reported inconsistent thickening performance under variable storage conditions.
The type of thickener is a known factor affecting the thickening process.22 One study using starch-based thickeners observed that the viscosity increased by 1.5 times in the first 10 minutes.23 Similarly, another study using a xanthan gum-based thickener reported that it took 45 minutes to reach the desired level of consistency in an infant formula.24 In our study, which used a thickener containing carob gum, the percentage of formulas reaching IDDSI Level 1 (Slightly Thick) increased over time: 27% at 15 minutes, 40% at 30 minutes, and 53% at 45 minutes. These findings indicate that the response of formulations to thickeners may vary significantly across different countries, and that resting time is a crucial factor in achieving the targeted IDDSI consistency level. Relying on standard practices without considering variables like formula type, temperature, storage conditions, and waiting time may be insufficient to provide safe and effective nutrition. Therefore, on-site viscosity assessments for each formulation are of great importance for the clinical decision-making process.
Frakking et al.19 reported that formulas with higher protein content were more likely to thicken, whereas formulas with higher sugar content were less likely to thicken. In our study, although we observed a trend of decreased thickening time as the amount of fat increased, this relationship was not statistically significant. In contrast to Frakking et al.’s findings, the macronutrient content in our study did not show a statistically significant relationship with the probability of thickening success. These findings suggest that under the tested preparation conditions, macronutrient composition alone may not be a reliable predictor of thickening outcomes for the evaluated starch-based thickener.
A study in Australia found that powdered formulas generally reached low to medium consistencies after 45 minutes, while liquid formulas showed greater variability.25 This aligns with the findings by Gosa and Choquett26, who also reported broad outcome variability. Our findings partially contradict Frakking et al.’s19conclusion that powdered formulas consistently perform better than liquids. These discrepancies may reflect differences in timing, thickener type (starch vs. gum), or environmental variables. These factors highlight the importance of product-specific evaluation and the need for broader standardization studies.
From a clinical standpoint, the inconsistency of thickening outcomes highlights the importance of on-site IDDSI flow testing before administration, particularly in settings where thickeners are used empirically. Additionally, the absence of manufacturer-specific guidance on rest time and the amount of thickener limits their effective clinical use. Even while following the commercial thickener’s instructions, only 53% of the formulas reached at least Level 1 at 45 minutes.
Limitations
This was a laboratory (in-vitro) study; IDDSI flow test results reflect rheological properties and should not be used as a direct proxy for clinical swallowing safety or aspiration risk. Generalisability is limited by the modest product sample (n=15) and the exploratory nature of the statistical models Evaluation of a single commercially available thickener, and testing under a single controlled condition. The tested thickener was selected because it is the only product in Türkiye approved for infants and children under three years of age; this strengthens contextual relevance but may limit applicability to other thickener types and brands. Larger, multi-centre studies comparing multiple thickener formulations and testing across varied environmental conditions are warranted.
Conclusion
This study demonstrates that the thickening outcomes of infant and child formulas varied between products but were not reliably predicted by formula type or macronutrient content. While the manufacturer’s instructions (1.7 g/100 mL with a 7-minute rest) were followed, approximately half of the products did not achieve IDDSI Level 1 consistency in more than half of the products tested. Importantly, the commercially available starch-based thickener evaluated in this study lacks clear manufacturer instructions regarding the appropriate resting time, amount of thickener, or expected IDDSI outcomes for different formula types. This absence of guidance limits the safe and effective use of thickeners in clinical practice. Our findings emphasize the urgent need for standardized, evidence-based thickening protocols tailored to individual formula characteristics to support safer feeding practices in paediatric dysphagia management.
Practical applications
This study provides the first data from Türkiye evaluating the thickening behaviour of paediatric formulas using a standardized IDDSI framework. The findings can assist clinicians, dietitians, and hospital nutrition services in selecting appropriate formulas for children with dysphagia, ensuring safer and more effective feeding strategies. Additionally, manufacturers of paediatric nutrition products may use this information to improve labelling, formulation, and instructions for use of thickeners to meet clinical texture standards.
Ethical approval
This study does not involve any human or animal testing.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Goday PS, Huh SY, Silverman A, et al. Pediatric feeding disorder: consensus definition and conceptual framework. J Pediatr Gastroenterol Nutr. 2019;68:124-129. https://doi.org/10.1097/MPG.0000000000002188
- Printza A, Sdravou K, Triaridis S. Dysphagia management in children: implementation and perspectives of flexible endoscopic evaluation of swallowing (FEES). Children (Basel). 2022;9:1857. https://doi.org/10.3390/children9121857
- Cichero JAY, Lam P, Steele CM, et al. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: the iddsi framework. Dysphagia. 2017;32:293-314. https://doi.org/10.1007/s00455-016-9758-y
- Umay E, Eyigor S, Giray E, et al. Pediatric dysphagia overview: best practice recommendation study by multidisciplinary experts. World J Pediatr. 2022;18:715-724. https://doi.org/10.1007/s12519-022-00584-8
- Wilson E, Simione M, Polley L. Paediatric oral sensorimotor interventions for chewing dysfunction: a scoping review. Int J Lang Commun Disord. 2021;56:1316-1333. https://doi.org/10.1111/1460-6984.12662
- Ortiz Pérez P, Valero-Arredondo I, Torcuato-Rubio E, Herrador-López M, Martín-Masot R, Navas-López VM. nutritional issues in children with dysphagia. Nutrients. 2024;16:1590. https://doi.org/10.3390/nu16111590
- Ortega O, Martín A, Clavé P. Diagnosis and management of oropharyngeal dysphagia among older persons, state of the art. J Am Med Dir Assoc. 2017;18:576-582. https://doi.org/10.1016/j.jamda.2017.02.015
- Rajati F, Ahmadi N, Naghibzadeh ZAS, Kazeminia M. The global prevalence of oropharyngeal dysphagia in different populations: a systematic review and meta-analysis. J Transl Med. 2022;20:175. https://doi.org/10.1186/s12967-022-03380-0
- Hirano I, Kahrilas PJ. Dysphagia. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill Education; 2018. Available at: https://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192012726 (Accessed on April 30, 2025).
- Kirby M, Noel RJ. Nutrition and gastrointestinal tract assessment and management of children with dysphagia. Semin Speech Lang. 2007;28:180-189. https://doi.org/10.1055/s-2007-984724
- Schindler A, Ginocchio D, Ruoppolo G. What we don’t know about dysphagia complications? Rev Laryngol Otol Rhinol (Bord). 2008;129(2):75-78.
- Dion S, Duivestein JA, St Pierre A, Harris SR. Use of Thickened liquids to manage feeding difficulties in infants: a pilot survey of practice patterns in canadian pediatric centers. Dysphagia. 2015;30:457-472. https://doi.org/10.1007/s00455-015-9625-2
- Madhoun LL, Siler-Wurst KK, Sitaram S, Jadcherla SR. Feed-thickening practices in nicus in the current era: variability in prescription and implementation patterns. J Neonatal Nurs. 2015;21:255-262. https://doi.org/10.1016/j.jnn.2015.07.004
- Rofes L, Arreola V, Mukherjee R, Swanson J, Clavé P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Aliment Pharmacol Ther. 2014;39:1169-1179. https://doi.org/10.1111/apt.12696
- Duncan DR, Larson K, Rosen RL. Clinical aspects of thickeners for pediatric gastroesophageal reflux and oropharyngeal dysphagia. Curr Gastroenterol Rep. 2019;21:30. https://doi.org/10.1007/s11894-019-0697-2
- Stevens M, O’Rourke S, Casto SC, Benedict J, Lundine JP. clinical focus: findings and clinical implications for thickening formula with infant cereal using the international dysphagia diet standardisation initiative flow test. Am J Speech Lang Pathol. 2022;31:1601-1610. https://doi.org/10.1044/2022_AJSLP-21-00298
- Dewar RJ, Joyce MJ. Time-dependent rheology of starch thickeners and the clinical implications for dysphagia therapy. Dysphagia. 2006;21:264-269. https://doi.org/10.1007/s00455-006-9050-7
- Koo JK, Narvasa A, Bode L, Kim JH. Through thick and thin: the in vitro effects of thickeners on infant feed viscosity. J Pediatr Gastroenterol Nutr. 2019;69:e122-e128. https://doi.org/10.1097/MPG.0000000000002470
- Frakking TT, Whillans C, Rogash C, David M. Properties of Australian thickened formulae for infants and children: influence of preparation and nutritional content on IDDSI properties. J Texture Stud. 2023;54:736-744. https://doi.org/10.1111/jtxs.12762
- International Dysphagia Diet Standardisation Initiative (IDDSI). Complete IDDSI Framework and Detailed Definitions v2.0. 2019.
- Ng V, Bogaardt H, Tzannes G, Collins S, Docking K. Thickened formulas used for infants with dysphagia: influence of time and temperature. Dysphagia. 2022;37:923-932. https://doi.org/10.1007/s00455-021-10353-w
- Rush OM, Bolland AC, Gosa MM. Effect of mixing method on resulting thickness of infant formula. J Texture Stud. 2021;52:57-70. https://doi.org/10.1111/jtxs.12566
- September C, Nicholson TM, Cichero JAY. Implications of changing the amount of thickener in thickened infant formula for infants with dysphagia. Dysphagia. 2014;29:432-437. https://doi.org/10.1007/s00455-014-9523-z
- Yoon SN, Yoo B. Rheological Behaviors of thickened infant formula prepared with xanthan gum-based food thickeners for dysphagic infants. Dysphagia. 2017;32:454-462. https://doi.org/10.1007/s00455-017-9786-2
- Marshall J, Buttsworth J, Grandt HDS, et al. Testing and development of slightly thick infant formula recipes for dysphagia management: an Australian perspective. Dysphagia. 2023;38:1254-1263. https://doi.org/10.1007/s00455-022-10550-1
- Gosa MM, Choquette CK. Effect of commercially available thickening agents on ready-to-feed infant formulas. J Texture Stud. 2021;52:612-622. https://doi.org/10.1111/jtxs.12600
Copyright and license
Copyright © 2026 The author(s). This is an open-access article under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.