Abstract
Objective: Parenteral nutrition (PN) is commonly used in patients with prolonged catabolic states when enteral feeding is not feasible. PN can be administered via pre-mixed multichamber bags (MCBs) or compounded bags (COBs). This study compares the biochemical, clinical effects, and complications of MCB and COB nutrition.
Methods: We retrospectively reviewed adult patients who received TPN in 2020 in an University Hospital. Patients were grouped based on receiving MCB or COB. Demographic data, lab values, hospital stay, and complications (hyperglycemia, refeeding syndrome, CRBSIs) were analyzed.
Results: A total of 235 patients were included. Hyperglycemia was more common in the MCB group, while CRBSI occurred more frequently in the COB group. COB patients more frequently met protein goals. Other biochemical and clinical outcomes were similar between groups.
Conclusion: MCB and COB both have unique advantages and drawbacks. Critically ill patients should be monitored closely regardless of PN formulation.
Keywords: parenteral nutrition, multichamber bags, compounded bags, critically ill, catheter-related infections
Main Points
- Both multichamber and compounded TPN methods are viable for critically ill patients.
- MCBs are associated with a higher risk of hyperglycemia.
- CRBSI is more frequent in COB group, influenced by infusion duration.
- No major differences were found in electrolyte or liver function outcomes.
Introduction
Parenteral nutrition (PN) is defined as the intravenous provision of nutrients via a central or peripheral vein. It may be used as the stand-alone nutrition support or as an adjunct to enteral nutrition.1 In patients severely ill with burns, acute pancreatitis, intestinal failure (such as extensive resection) and other reasons preventing adequate oral or enteral nutrition, PN prevents severe malnutrition and associated morbidity/mortality.2
There are two main types of parenteral nutrition preparation methods: hospital pharmacy–compounded bags (COBs) and commercial multichamber bags (MCBs). The COB can be standardized or tailored to the patient’s specific needs.3 As in our hospital, larger institutions with high acuity may use automated compounding devices to compound PN. These devices are used to customize a PN prescription for each patient. Due to limited stability of the compounded mixtures, COBs should be prepared every 24–48 hours in the case of inpatients requiring a daily delivery of PN; however, the exact frequency depends on the pharmacy service workload and on prescription changes. The commercial parenteral nutrition, known as “premixed or multichamber” PN, is a manufactured compounded, sterile product available in peripheral and central line formulations. In contrast to COBs, the shelf life of the MCBs is 12–24 months at room temperature.4,5 For patients without relevant comorbidities, multi chamber bags as standard parenteral nutrition mixtures are often adequate to correct nutrient deficiencies and their related complications.6 However, for patients with particular comorbidities (heart failure, chronic renal failure, hepatic failure), as well as for critically ill patients or for patients with benign chronic intestinal failure, compounded bags are often required.7 Yet, there is no consensus on the clinical advantages and disadvantages of different PN preparation methods. During the management of certain shortages in compounded total parenteral nutrition (TPN), institutions like ours have utilized MCBs in place of COBs.8,9
This retrospective study aims to compare hospitalized patients’ clinical data, biochemical values as serum electrolytes, blood glucose levels, prealbumin, kidney and liver function tests, length of hospital stay (LOS) and PN complications while they are receiving PN regimens as either MCBs or COBs. We hypothesized that patients who used different PN regimens (as MCBs or as COBs) had different risk of biochemical derangements, complications, different LOS and other clinical outcomes.
Materials and Methods
After ethical approval from the Hacettepe University Faculty of Medicine Ethics Committee (GO 21/272, 21.05.2021) files of all patients who had received PN products in Hacettepe University Adult Hospital in 2020, were analyzed retrospectively. We included all adult hospitalized patients who had been on PN consecutively for at least 3 days.
In our institution, patients needing PN must be evaluated by a specialized nutritional team to establish their fluid, electrolytes, and macro and micronutrient requirements. Therapeutic decisions on using PN are taken by the attending physicians and the hospital nutrition support team (NST). Our NST consists of physicians (geriatricians, internists, surgeons, anesthesiologists, oncologists, gastroenterologists, pediatricians), dietitians, nurses, and pharmacists. After hospitalization, all patients are routinely screened for malnutrition with Nutritional risk screening (NRS 2002)10 by the attending physician. The patients at malnutrition risk (NRS 2002≥4) or have contraindications to oral/enteral nutrtion (even if NRS 2002<4) are consulted to the NST for further assessment of their nutrition status. The contraindications to enteral nutrition are intestinal failure, intraabdominal infections, postoperative ileus, intestinal obstruction, severe burns, multiple trauma, or high output intestinal fistula. While providing nutrition assessment and determining nutritional needs, the NST aims to ensure appropriate and safe nutritional support to each patient. NST recommends oral or enteral nutrition initially. However, if the oral/enteral intake is inadequate or contraindicated, supplementary or total PN is recommended. Daily nutritional requirements were calculated as 20-30 kcal/kg/day for energy requirements and 1.2-2 g/kg/day for protein requirements. The patients who received a consultation with the NST for PN therapy for the first time were included into this study, while those who were already under nutritional therapy were excluded. Data about nutrition goals and the amount of nutrition actually received as well as PN associated complications were retrieved from NST files and electronic patient files.
All PN ordered were prepared by NST guided ACD (EM 2400 (Exacta Mix) automated compounding device to compound PN with aseptic technique. Patients were grouped according the PN product type they received: COBs or MCBs. The COB solution was prepared in a 2000-mL TPN bag (TPN EVA Bag, Kapsam Medical) with 10% Freselamin ( Osel Pharmaceuticals), 20% Clinoleic (Baxter S.A.), 20%/30% dextrose, 0.9%/3% NaCl, 7.5% KCl, 10% Ca Picken (ADEKA Pharmaceuticals), 15% MgSO4, multivitamins (Todavit, Polifarma), and trace elements (Addamel N20, Fresenius Kabi). MCBs used were Oliclinomel N-4 550 E (Baxter S.A.), Oliclinomel N7-1000 E (Baxter S.A.), and Kabiven Peripheral (Fresenius Kabi). Multivitamins and trace elements were added into the MCBs with aseptic technique.
Patients’ demographic data, comorbidities, causes of hospital admission, NRS 2002 score, Intensive Care Unit (ICU) stay, duration of hospital stay, survival status, serum sodium (normal levels 136-146 mEq/L), potassium (normal levels 3.5-5.1 mEq/L), magnesium (normal levels 1.8-2.5 mg/dL), chloride (normal levels 101-109 mEq/L), inorganic phosphorus (normal levels 2.5-4.5 mg/dL) and blood glucose levels (normal levels 70-100 mg/dL), albumin (normal levels 3.5-5.2 g/dL), prealbumin, creatinine (normal levels 0.51-0.95 mg/dL), blood urea nitrogen (normal levels 6-20 mg/dL) levels and liver function tests, and catheter related complications (catheter thrombosis, line obstruction or accidental removal of the catheter) were recorded. Data for the study were derived from Nucleus database of Hacettepe University Adult Hospital. Besides abnormal electrolyte levels, other PN related metabolic complications including hyperglycemia, refeeding syndrome, hyperlipidemia, hepatic disorders were observed after the administration of parenteral nutrition (PN). Hyperglycemia was defined as random blood glucose over 200 mg/dL11 while on TPN infusion. Since refeeding syndrome can be defined as the potentially fatal shifts in fluids and electrolytes that may occur in malnourished patients receiving artificial refeeding, we set refeeding syndrome limits as hypophosphatemia, with a fall from baseline greater than 30% or more than 0.16mmol/l.12 Hypertriglyceridemia was defined as plasma levels above 200 mg/dL. In accordance with a previous study13, liver dysfunction (LD) was defined as: Cholestasis: alkaline phosphatase (ALP) > 280UI/L, gamma-glutamyltransferase (GGT) > 50UI/L and total bilirubin (TB) > 1.2 mg/dL; Hepatic necrosis: Aspartate aminotransferase (AST) > 40UI/L, alanine aminotransferase (ALT) > 42UI/L and Mixed pattern: ALP > 280UI/L, GGT > 50UI/L or TB > 1.2 mg/dL plus AST > 40 IU/L or ALT > 42UI/L.
Catheter related complications included phlebitis, catheter exit site infection, bacteremia and catheter related bloodstream infection (CRBSI) as defined by Infectious Diseases Society of America (IDSA). A definitive diagnosis of CRBSI required that the same organism grow from at least one percutaneous blood culture and from a culture of the catheter tip, or growth of microbes from the blood sample drawn from a catheter hub at least 2 hours before microbial growth is detected in a blood sample obtained from the peripheral vein.14
All analyses were performed using SPSS (Statistical Package for Social Sciences, version 22.0). Student’s t-test was used to compare groups in terms of normally distributed quantitative variables (age, height, weight). Mann-Whitney U-tests were used to compare the groups in terms of nonparametric data. Normally distributed parametric data were presented as mean ± standard deviation (mean ± SD) and non-parametric data as median (minimum-maximum) values. The chi-square test was used to compare categorical data between the groups. The time dependent within group and between group analysis was performed by general linear model for repeated measures analysis. Parameters that were statistically significant at univariate analysis were included in multivariate logistic regression analysis. p<0.05 values were considered statistically significant.
Results
When the files of 239 patients who received parenteral nutrition in the Hacettepe Adult Hospital in 2020 were scanned, 4 patients were found to have missing data. Therefore, 235 patients were included in the study. Finally, Group I was constituted from 190 patients with COBs received, while Group II was constituted from 165 patients with MCBs received.
The baseline characteristics of the patients in the two groups are listed in Table 1. Moreover, protein and calorie goals per day, indications and duration of infusions of the two types of PN are presented in Table 2. There were no statistically significant differences in both groups regarding the indications to start and to stop the PN, the protein and calorie goals of nutrition and duration of the PN infusion. Patients received more proteins and hence, the nutrition protein goals were reached more commonly with COBs when compared to MCBs.
| Data is given as n (%), mean±SD or median [minimum-maximum] as appropriate. | |||
| Table 1. Demographics and clinical characteristics of the two groups of patients who received different types of TPN (Compounded Parenteral Nutrition vs Pre-mixed Multichamber Bags) | |||
| Parameter |
|
|
|
| Age, years |
|
|
|
| Gender, M/F |
|
|
|
| Height (cm) |
|
|
|
| Weight (kg) |
|
|
|
| NRS 2002 |
|
|
|
| Comorbidities: | |||
| Diabetes Mellitus |
|
|
|
| Chronic Obstructive Pulmonary Disease |
|
|
|
| Chronic Renal Failure |
|
|
|
| Coronary Artery Disease |
|
|
|
| Congestive Heart Failure |
|
|
|
| Hypertension |
|
|
|
| Causes of hospital admission: | |||
| Infection |
|
|
|
| Neurologic |
|
|
|
| Gastrointestinal |
|
|
|
| Cancer |
|
|
|
| ICU patients |
|
|
|
| Duration of ICU stay (days) |
|
|
|
| Duration of hospital stay (days) |
|
|
|
| Mortality |
|
|
|
|
EN: Enteral nutrition IJV: Internal jugular vein, SCV: subclavian vein, PN: Parenteral nutrition, TPN: Total parenteral nutrition. Data is presented as n (%) or median [minimum-maximum] and the two groups were compared with Chi-Square tests or Mann-Whitney U tests, respectively. |
|||
| Table 2. Indications and clinical properties of the two types of PN | |||
| Parameter |
|
|
|
| PN indication: | |||
| Neurologic causes |
|
|
|
| Intestinal obstruction |
|
|
|
| Perioperative support |
|
|
|
| Intraabdominal infection |
|
|
|
| Insufficient oral/EN intake |
|
|
|
| EN during TPN |
|
|
|
| EN goal (kcal/day) |
|
|
|
| EN protein goal (mg/day) |
|
|
|
| EN calories received (kcal/day) |
|
|
|
| EN protein received (mg/day) |
|
|
|
| EN calorie goal reached, n (%) |
|
|
|
| Central /peripheral TPN |
|
|
|
| Type of the central catheter: | |||
| Port/Hickman/IJV/SCV |
|
|
|
| PN calorie goal (kcal/day) |
|
|
|
| PN protein goal (mg/day) |
|
|
|
| PN calories received (kcal/day) |
|
|
|
| PN protein received (mg/day) |
|
|
|
| Duration of TPN infusion (days) |
|
|
|
| Reason to stop PN: | |||
| Oral nutrition |
|
|
|
| Discharged from the hospital |
|
|
|
| Home enteral nutrition |
|
|
|
| Home TPN |
|
|
|
| Clinical deterioration |
|
|
|
There was no significant difference between the groups regarding PN related mechanical complications (Table 3). There were no cases of catheter thrombosis, line obstruction or accidental removal of the catheter. PN related metabolic complications were also similar in the two groups except MCBs were associated with more hyperglycemia than COBs. When the group of patients with hyperglycemia was compared with the group of patients without hyperglycemia the differences other than the type of PN were the presence of diabetes (71% vs 14%, p=0.001), chronic renal failure (29% 3%, p=0.022), congestive heart failure (29% vs 4%, p=0.030). When the grouping variable and the presence of diabetes, chronic renal failure and congestive heart failure were entered into binary logistic regression analysis of the hyperglycemia as the dependent variable; the presence of diabetes (B=2.479, S.E.=0.959, p=0.010), and chronic renal failure (B=2.451, S.E.=1.157, p=0.034) were the statistically significant independent variables whereas the grouping variable (COB or MCB) (p=0.994) and congestive heart failure (p=0.523) were not statistically significant variables.
| Table 3. Complications of PN in the two groups | |||
|
|
|
|
|
| Insulin infusion required, n (%) |
|
|
|
| PN Hyperglycemia, n (%) |
|
|
|
| PN Hypertriglyceridemia, n (%) |
|
|
|
| PN Hypernatremia, n (%) |
|
|
|
| PN Hypophosphatemia, n (%) |
|
|
|
| PN Hypopotassemia, n (%) |
|
|
|
| Refeeding syndrome, n (%) |
|
|
|
| Liver dysfunction: | |||
| Cholestasis, n (%) |
|
|
|
| Hepatic necrosis, n (%) |
|
|
|
| Mixed pattern, n (%) |
|
|
|
| Phlebitis, n (%) |
|
|
|
| Catheter insertion site infections, n (%) |
|
|
|
| Bacteremia, n (%) |
|
|
|
| CRBSI, n (%) |
|
|
|
The only difference in PN related infectious complications between the two groups was the higher incidence of CRBSI in the COBs group. The most common microorganism associated with CRBSI was Candida albicans (6 cases), followed by Candia parapsilosis (3 cases) and Staphylococcus aureus (2 cases). When the group of patients with CRBSI was compared with the group of patients without CRBSI the only difference other than the type of PN was the duration of infusion (29[5-120] days vs 11[1-277] days, respectively) (Mann- Whitney U test, p<0.001). When the grouping variable and the duration of infusion were entered into binary logistic regression analysis of the CRBSI as the dependent variable; duration of infusion was the only statistically significant independent variable (B=0.018, S.E.=0.004, p=0.041) whereas the grouping variable (COB or MCB) was not (p=0.996).
Furthermore, patients’ biochemical parameters are monitored at the initial day and 3, 7, 14, 21, 28 days after the start of PN nutrition (Figure 1). The time dependent within group and between group analysis was performed by general linear model for repeated measures analysis, which revealed statistically similar trends in the two groups regarding sodium, potassium, chloride, magnesium, ALT, AST, ALP, GGT, total bilirubin, BUN, creatinine, albumin and prealbumin levels as shown in Figure 1. Of all the biochemical follow up measurements only the trends of the inorganic phosphorus levels were different between the two groups (p=0.002 for tests of between subject effects).
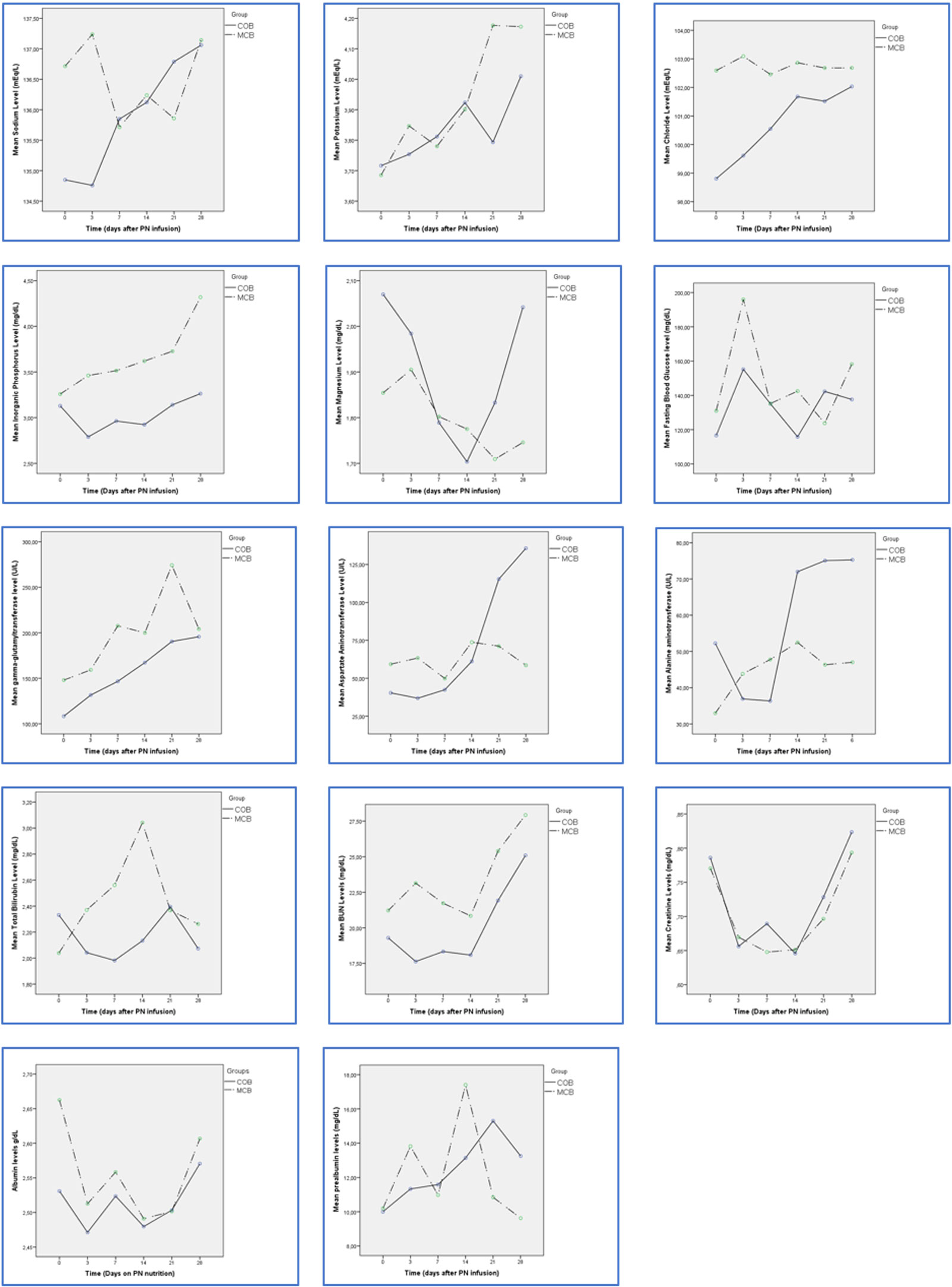
When subgroup analysis was performed for the ICU patients (n=168 total, 87 patients received COB, 81 patients received MCB), the diabetic, hypertensive, chronic heart failure patients, and hyperglycemia was more common with MCBs (25%, 36%, 10% and 6% respectively) when compared to COBs (12%, 17%, 2% and 0% respectively) whereas CRBSI was more common with COBs (6%) compared to MCBs (0%). In critically ill patients, COB PN was infused longer (median 14[2-277] days) than MCB PN (median 11[1-131] days) (p=0.009). Although the durations of ICU stay were similar (median 8[1-104] days in the COB group versus median 7[1-64] days in the MCB group) in these two subgroups, length of hospital stay was longer in the COB group than the MCB group (median 39[4-455] days and (median 26[4-143] days, respectively, P=0.004).
Discussion
In our study, we aimed to compare safety of two parenteral nutrition regimens (MCB vs COB) regarding the efficacy of nutrition support avoiding clinical side effects in hospitalized patients.
Despite the perceived benefits of premixed multichamber bag solutions, many hospitals have been slow to use them because they can’t be tailored or customized to meet patients’ individual medical needs. Multichamber bags tend to contain less protein and fewer electrolytes such as sodium, potassium, chloride, and acetate compared with personalized compounded solutions.4 Our study confirmed that patients received less proteins and hence, the nutrition protein goals were reached less commonly with MCBs when compared to COBs probably due to the fact that COBs were preferred more commonly as central infusions than MCBs (47% vs 24% respectively). On the other hand, the trends in serum electrolytes were quite similar with these two types of PN. This can be explained by the close supervision and necessary replacement of the serum electrolytes by the physicians.
Although PN is an effective method of nutrition support, it has been associated with a range of mechanical, septic, and metabolic complications. Hyperglycemia is found in up to 50% of PN patients. In addition to long term complications, even short-lasting blood glucose values over 200 mg/dL appear unacceptable because they interfere with quality of life by inducing dehydration and polyuria.15 In our study, almost 80% of patients in both groups received insulin infusion resulting in low incidence of hyperglycemia. All the TPN associated hyperglycemia cases were seen in the patients who received MCB PN. The presence of diabetes and chronic renal failure were found to be independent predictors of PN hyperglycemia. These two groups of patients may benefit from lower dextrose containing PN such as in COB.
Refeeding syndrome (RS) consists of a group of clinical signs and symptoms that occur in malnourished patients receiving nutrition support after a long fasting period. These signs and symptoms include electrolyte disorders, especially a reduction in intracellular electrolytes (potassium, magnesium, and phosphorus); altered glucose metabolism (hyperglycemia); and a deficiency of vitamins and oligoelements.12,16,17 A systematic review and meta-analyses of literature reported an incidence of RS varying from 7% to 62% depending on the definition used and the population studied.18 In our study, we found RS in 60% of our patients on PN. More than half of our patients had malnutrition (NRS 2002≥4), and more than half were in the ICU both of which are high risk factors for the development of RS. The high prevalence of patients with RS receiving PN highlights the need for the development of strategies for prevention and adequate nutrition approach for this population, which would require the implementation of specific protocols in the hospitals. Friedli et al.19 proposed an evidence-based algorithm for the management and prevention of RS that encompasses the identification, prevention, management, and monitoring of RS, and it could be adapted for each PN team according to the particularities of the service and patients. In our study, refeeding incidence was similarly high in both groups (MCBs and COBs).
Hypertriglyceridemia is found in approximately 25–50% of PN patients.1 One should aim for plasma triglyceride concentrations below 400 mg/dL during PN infusion. Severe hypertriglyceridemia (>1000 mg/dL and particularly >5000 mg/dL) can induce acute pancreatitis, similar to patients with severe hypertriglyceridemia without PN, and it can affect micro circulation.20 In our study, 3% of our patients had elevated triglyceride levels and the type of the PN (MCBs or COBs, both of which contained olive oil) did not affect the triglyceride levels.
One of the other common complications of parenteral nutrition is LD, which is associated with a higher risk of mortality.20 However, the causes of hepatic and biliary abnormalities induced by continuous PN have not been identified yet20 even patients who receive PN for a short time frequently develop cholestasis.21 Hepatic parameters should be continuously monitored in patients receiving PN to prematurely detect and treat any potential liver dysfunction. For this reason, our protocol included third day and the weekly monitoring of hepatic parameters. In both COB and MCB groups liver function tests were similar and tended to elevate as time passes on PN.
The use of the serum protein levels for nutritional assessment is well established. The relationship of serum albumin concentration ≤ 3.5 g/dl to an increased morbidity and mortality in medical and surgical patients is well documented.22,23 However, it has also been suggested that a biochemical assessment of albumin is not a reliable marker of the nutrition status. The albumin concentrations slowly respond to protein restriction and are more a reflection of the patient’s illness than the nutritional intake. Prealbumin responds quickly to the onset of malnutrition and rises rapidly with the adequate protein intake. Several studies have reported that patients with low prealbumin levels have a shorter length of stay in hospital stay and fewer complications, lower morbidity and possible mortality, if they are given either intravenous or oral hyperalimentation.24,25 A prospective randomized study in five Chinese hospitals compared MCB parenteral nutrition to customized PN formulations. Among 240 patients, prealbumin levels rose more dramatically in the patients who received MCBs than the patients who received COB, but this difference could be attributed to the different types of the lipid compositions used in the two groups studied.26 Reliability of prealbumin as a biomarker for nutrition state, however is also limited since prealbumin is a negative acute phase protein and serum levels are influenced not only by nutrition but also by the inflammatory state. However, prealbumin is still commonly used due to its small pool size and short half life.27 We also monitored patients’ prealbumin levels while they received TPN and found no statistical difference between the two groups (MCB vs COB).
In our study, there was no documented CRBSI among the patients in MCB group but 6% of patients had CRBSI in the COB group. The higher use of central catheters may explain the higher CRBSI in the COB group. The duration of PN infusion was the only statistically significant independent variable of CRBSI. Another study reported bloodstream infection percentage of 6.8% in the COB group, quite similar to our COB group (6%) whereas it reported a higher ratio of bloodstream infection (5.6%) in the MCB groups than our MCB group.28
The main limitation of our study is its retrospective design and that not all relevant variables were available in the electronic health record. On the other hand, it is worth mentioning that PN and follow-up data were registered prospectively by the nutrition support team, which minimized measurement bias. Close supervision by the nutrition support team helped earlier detection and proper management of complications in both types of PN (COB and MCB).
In line with the notion of standardization of medications/formulations whenever possible to improve patient safety, multichamber bag solutions are closed systems premade by a manufacturers and are considered low risk for harboring contaminants and bacteria.5,29,30 Moreover, compounding, labeling, and administration errors are significantly higher when hospital staff use compounded bags vs premixed multichamber bag solutions. As a result, the American Society for Parenteral and Enteral Nutrition (ASPEN) established best practices for compounding PN and determined that premixed formulations or multichamber bag solutions would be valuable alternatives.8
We conclude that MCBs and COBs have both different pros and cons in terms of clinical outcomes; specifically, critically ill patients should be closely monitored for the effects of parenteral nutrition with either MCB or COB. MCB can be an alternative to COB with vigilant monitoring and appropriate management of metabolic complications, especially hyperglycemia. Patients with diabetes and chronic renal failure may benefit more with COB as dextrose content can be adjusted. It should be noted that specific illnesses such as renal failure, liver dysfunction, electrolyte imbalances, and patients with increased and /or spesific metabolic needs may also benefit more from personalized compounded solutions compared to premixed solution.
Acknowledgements
The authors thank the Clinical Nutrition Team at Hacettepe University Adult Hospital for their contributions.
Ethical approval
This study has been approved by the Hacettepe University Faculty of Medicine Ethics Committee (approval date 21.05.2021, number GO 21/272). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- American Society for Parenteral and Enteral Nutrition. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26(Suppl 1):1SA-138SA. https://doi.org/10.1177/0148607102026001011
- Mercaldi CJ, Reynolds MW, Turpin RS. Methods to identify and compare parenteral nutrition administered from hospital-compounded and premixed multichamber bags in a retrospective hospital claims database. JPEN J Parenter Enteral Nutr. 2012;36:330-336. https://doi.org/10.1177/0148607111412974
- Berlana D, Almendral MA, Abad MR, et al. Cost, time, and error assessment during preparation of parenteral nutrition: multichamber bags versus hospital-compounded bags. JPEN J Parenter Enteral Nutr. 2019;43:557-565. https://doi.org/10.1002/jpen.1436
- Beattie C, Allard J, Raman M. Comparison between premixed and compounded parenteral nutrition solutions in hospitalized patients requiring parenteral nutrition. Nutr Clin Pract. 2016;31:229-234. https://doi.org/10.1177/0884533615621046
- Bozat E, Korubuk G, Onar P, Abbasoglu O. Cost analysis of premixed multichamber bags versus compounded parenteral nutrition: breakeven point. Hosp Pharm. 2014;49:170-176. https://doi.org/10.1310/hpj4902-170
- Braga M, Ljungqvist O, Soeters P, et al. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378-386. https://doi.org/10.1016/j.clnu.2009.04.002
- Pironi L, Goulet O, Buchman A, et al. Outcome on home parenteral nutrition for benign intestinal failure: a review of the literature and benchmarking with the European prospective survey of ESPEN. Clin Nutr. 2012;31:831-845. https://doi.org/10.1016/j.clnu.2012.05.004
- Boullata JI, Gilbert K, Sacks G, et al. ASPEN clinical guidelines: parenteral nutrition ordering, order review, compounding, labeling, and dispensing. JPEN J Parenter Enteral Nutr. 2014;38:334-377. https://doi.org/10.1177/0148607114521833
- Bonnes SL, Austin KE, Carnell JJ, Salonen BR. Premixed vs Compounded Parenteral Nutrition: Effects of Total Parenteral Nutrition Shortage on Clinical Practice. Curr Nutr Rep. 2019;8:397-401. https://doi.org/10.1007/s13668-019-00291-3
- Kondrup J, Rasmussen HH, Hamberg O, Stanga Z; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321-336. https://doi.org/10.1016/s0261-5614(02)00214-5
- Cook CB, Kongable GL, Potter DJ, et al. Inpatient glucose control: a glycemic survey of 126 U.S. hospitals. J Hosp Med. 2009;4:E7-E14. https://doi.org/10.1002/jhm.533
- Boot R, Koekkoek KWAC, van Zanten ARH. Refeeding syndrome: relevance for the critically ill patient. Curr Opin Crit Care. 2018;24:235-240. https://doi.org/10.1097/MCC.0000000000000514
- Grau T, Bonet A, Rubio M, et al. Liver dysfunction associated with artificial nutrition in critically ill patients. Crit Care. 2007;11:R10. https://doi.org/10.1186/cc5670
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1-45. https://doi.org/10.1086/599376
- Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041-2047. https://doi.org/10.1001/jama.290.15.2041
- Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336:1495-1498. https://doi.org/10.1136/bmj.a301
- Gómez-Tello V. Nutritional assessment of the severely ill patient. Nutr Hosp. 2005;20:5-8.
- Cioffi I, Ponzo V, Pellegrini M, et al. The incidence of the refeeding syndrome. A systematic review and meta-analyses of literature. Clin Nutr. 2021;40:3688-3701. https://doi.org/10.1016/j.clnu.2021.04.023
- Friedli N, Stanga Z, Culkin A, et al. Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition. 2018;47:13-20. https://doi.org/10.1016/j.nut.2017.09.007
- Hartl WH, Jauch KW, Parhofer K, Rittler P; Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. Complications and monitoring - Guidelines on Parenteral Nutrition, Chapter 11. Ger Med Sci. 2009;7:Doc17. https://doi.org/10.3205/000076
- Angelico M, Della Guardia P. Review article: hepatobiliary complications associated with total parenteral nutrition. Aliment Pharmacol Ther. 2000;14(Suppl 2):54-57. https://doi.org/10.1046/j.1365-2036.2000.014s2054.x
- Spiekerman AM. Nutritional assessment (protein nutriture). Anal Chem. 1995;67:429R-436R. https://doi.org/10.1021/ac00108a026
- Georgiannos S, Renaut A, Goode A. Short-term restorative nutrition in malnourished patients: pro’s and con’s of intravenous and enteral alimentation using compositionally matched nutrients. Int Surg. 1997;82:301-306.
- Johnson AM. Low levels of plasma proteins: malnutrition or inflammation? Clin Chem Lab Med. 1999;37:91-96. https://doi.org/10.1515/CCLM.1999.017
- Birnvenu K, Jeppsson J, Inglebleek Y, et al. Serum proteins in clinical medicine. 1996.
- Yu J, Wu G, Tang Y, Ye Y, Zhang Z. Efficacy, Safety, and Preparation of Standardized Parenteral Nutrition Regimens: Three-Chamber Bags vs Compounded Monobags-A Prospective, Multicenter, Randomized, Single-Blind Clinical Trial. Nutr Clin Pract. 2017;32:545-551. https://doi.org/10.1177/0884533617701883
- Torgersen Z, Balters M. Perioperative nutrition. Surg Clin North Am. 2015;95:255-267. https://doi.org/10.1016/j.suc.2014.10.003
- Banko D, Rosenthal N, Chung J, Lomax C, Washesky PF. Comparing the risk of bloodstream infections by type of parenteral nutrition preparation method: A large retrospective, observational study. Clin Nutr ESPEN. 2019;30:100-106. https://doi.org/10.1016/j.clnesp.2019.01.011
- Pichard C, Schwarz G, Frei A, et al. Economic investigation of the use of three-compartment total parenteral nutrition bag: prospective randomized unblinded controlled study. Clin Nutr. 2000;19:245-251. https://doi.org/10.1054/clnu.2000.0106
- Singh A, Rauch D. Commercial premixed parenteral nutrition and its potential role in pediatrics. Hosp Pediatr. 2016;6:34-36. https://doi.org/10.1542/hpeds.2015-0147
Copyright and license
Copyright © 2025 The author(s). This is an open-access article under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.










