Abstract
Background: Parenteral Nutrition (PN) is a life-sustaining therapy for patients with intestinal failure (IF), especially those with severe bowel dysmotility. Home Parenteral Nutrition (HPN) can reduce hospital stays and improve quality of life. While adult-designed commercial multichamber parenteral nutrition bags (AMCB-PN) are widely used in adults, their application in pediatric patients remains underexplored.
Case Presentation: We report a male patient born in 2008 with bowel dysmotility and malabsorption, requiring long-term PN since infancy. After initial stabilization with a 2-in-1 PN regimen, he was discharged with HPN at one year of age. Despite attempts to wean off PN, he continued to require partial PN support. In 2019, the patient transitioned from a 2-in-1 PN system to a standardized AMCB-PN regimen. This change significantly reduced caregiver burden by eliminating frequent lipid syringe changes and minimizing infusion pump requirements. Over five years of AMCB-PN use, the patient maintained stable weight gain, demonstrated good tolerance to oral intake, and remained free of major complications.
Discussion: This case demonstrates the feasibility and safety of using AMCB-PN in a pediatric patient with chronic intestinal failure. Benefits included improved sleep quality, reduced infection risk, simplified administration, and cost savings of approximately 52% annually. Despite the patient being physically smaller than age norms, he remained active with improved quality of life.
Conclusion: Standardized AMCB-PN can be a safe, cost-effective, and patient-friendly alternative to in-house compounded PN in selected pediatric patients, offering significant clinical and operational advantages in home-based care.
Keywords: parenteral nutrition, home parenteral nutrition, multichamber bag, pediatric intestinal failure, AMCB-PN, case report
Main Points
- Long-term home parenteral nutrition (HPN) was successfully managed in a pediatric patient with bowel dysmotility using initially a 2-in-1 PN formulation, transitioning later to adult-designed commercial multichamber PN bags (AMCB-PN).
- Switching from 2-in-1 PN to AMCB-PN improved safety and convenience by reducing the frequency of lipid syringe changes, lowering infection risk, and simplifying infusion with a single pump.
- The use of adult-designed multichamber PN bags in pediatric patients, although traditionally considered nutritionally inadequate, was demonstrated to be feasible, safe, and effective in this selected case.
- The transition to AMCB-PN resulted in significant cost savings (approximately 52% reduction in annual costs) by decreasing consumable use and pump maintenance expenses.
- Multidisciplinary Nutrition Therapy Teams (NTTs) played a critical role in optimizing PN regimens, monitoring patient progress, and ensuring safe home administration over many years.
Introduction
Parenteral Nutrition (PN) is an intravenous administration method that delivers essential macronutrients, electrolytes, and micronutrients directly into the bloodstream, bypassing the digestive system entirely.1 It is essential for the management of patients with intestinal failure (IF). Severe protracted intestinal failure can result from short bowel syndrome, congenital diseases, and severe intestinal motility disorders. In these chronic scenarios, long-term parenteral nutrition is often necessary to sustain life.2 For those requiring prolonged PN support, Home Parenteral Nutrition (HPN) offers the potential to reduce hospital stays and improve the quality of life for both patients and caregivers.1
The latest ESPGHAN recommendations endorse the use of standard licensed multichambered bag for patients of the same age group with similar conditions. This approach aims to enhance safety, improve stability, reduce time pressure, ensure better quality control, and optimize resources for better cost-effectiveness while maintaining the quality of care.3 Individualized PN formulations should only be considered when daily prescription changes are necessary according to the patient’s past medical condition and the latest laboratory tests, or when standardized formulas are unable to meet the requirements.3 With advancements in technology, the use of multichambered or all-in-one (AIO) standardised PN solution bags has proven to be both feasible and safe for use.4,5 While limited research is available on the use of adult-designed commercial multichambered parenteral nutrition bags (AMCB-PN) in paediatric patients between 2 to 18 years of age, this case underscores the potential for safe and effective application of a standard AMCB-PN within the paediatric population.
Case Presentation
A male patient was born on September 2008 at Hospital Tunku Jaafar with body weight 3.6kg. At 4 months of age, the child was referred from Klinik Kesihatan Beranang to Hospital Kajang due to persistent vomiting and inadequate weight gain, with the child’s weight remaining stagnant at 4 kg. Subsequently, he was transferred from Hospital Kajang to Hospital Tunku Azizah, previously known as Institut Pediatrik Hospital Kuala Lumpur, for further evaluation of abdominal distension, vomiting, diarrhoea, and continued poor weight gain. On January 2009, inborn errors of metabolism (IEM) screening were conducted and the result come back as normal, however, a subsequent rectal biopsy was performed, and the finding was ganglionic.
A laparotomy, ileostomy and small bowel biopsy were performed in January 2009. During the operation, the finding was dilated small intestines with edematous appearance and lacteal appearance on the outer surface. His small intestines length is 92cm with no evidence of malrotation or adhesion while large intestines and appendix were found normal. Result of the histopathological examination (HPE) then revealed ganglionic small intestine, ileum and no intramucosal or submucosal dilated lymphatic.
The patient was diagnosed with malabsorption secondary to bowel dysmotility. Parenteral nutrition using a 2-in-1 formulation was initiated in January 2009 via a peripherally inserted central catheter (PICC). Throughout his hospitalization, he was closely monitored for nutritional intake, fluid and electrolyte balance, and catheter care, in accordance with clinical protocols.
In March 2009 the patient underwent bishop-koop procedure and insertion of central venous lines (CVL). Intraoperatively, a prolapsed proximal ileum was seen. HPE result shown morphological appearance on both proximal ileostomy end and distal ileostomy end with no significant inflammation on the appendix.
In September 2009, the HKL team has come to an agreement that the nutritional requirements and the PN regimen had been stabilized and optimized. He was discharged from HKL at the age of 1 years old with home parenteral nutrition (HPN) at the frequency of 3 times per week with duration of 18-hour infusion. Prior to discharge, the patient’s mother underwent 3 week in-ward training and demonstrated competence in HPN management, including line care and the preparation of enteral and parenteral nutrition using strict aseptic technique. His family also has made all the necessary adjustment and renovation of their house in Beranang, Selangor ensuring they have a dedicated room that meets safety standards and is suitable for PN administration, in accordance with the guidelines.
A bowel motility study conducted at the age of 4 demonstrated normal findings. The team then decided to reduce PN and increase oral feeding and subsequently able to off PN in September 2012. However, after 2 weeks his weight shows reducing in trend with his current weight is 11.6kg. He was then started back on same PN regimen with frequency of 3 times per week.
With advancing age, the patient’s PN regimen was adjusted annually to ensure adequate nutrient delivery based on age and weight specific requirements. Summary of patient’s PN regimen was tabulated in Table 1. As of 2024, at 16 years of age, the patient’s estimated energy requirement was approximately 30-55kcal/kg/day.6 Discussions have been made by healthcare professional regarding his parenteral nutrition treatment including the use of standard AMCB-PN. Even though changing from 2-in-1 PN to AMCB-PN is safe and feasible, 2-in-1 PN still the most preferred PN among healthcare professional as well as patient caretaker.
| Table 1. Summary of parenteral nutrition regimen delivered from 2011 until 2024 | |||||||
| PN Contents |
|
|
|
|
|
|
|
| Macronutrient | |||||||
| Amino acids (g/kg/day) |
|
|
|
|
|
|
|
| Glucose (g/kg/day) |
|
|
|
|
|
|
|
| Fat (g/kg/day) |
|
|
|
|
|
|
|
| Electrolytes | |||||||
| Sodium (mmol/kg/day) |
|
|
|
|
|
|
|
| Potassium (mmol/kg/day) |
|
|
|
|
|
|
|
| Calcium (mmol/kg/day) |
|
|
|
|
|
|
|
| Magnesium (mmol/kg/day) |
|
|
|
|
|
|
|
| Phosphate (mmol/kg/day) |
|
|
|
|
|
|
|
| Micronutrient | |||||||
| Water soluble vitamins (ml/day) |
|
|
|
|
|
|
|
| Fat soluble vitamins (ml/day) | |||||||
| Trace elements (ml/day) |
|
|
|
|
|
|
|
| Total Volume (ml/kg/day) |
|
|
|
|
|
|
|
| Total Calorie (kcal/kg/day) |
|
|
|
|
|
|
|
| Duration of PN infusion (hour) |
|
|
|
|
|
|
|
| Type of PN bag |
|
||||||
| PN Contents |
|
|
|
|
|
|
|
| Macronutrient | |||||||
| Amino acids (g/kg/day) |
|
|
|
|
|
|
|
| Glucose (g/kg/day) |
|
|
|
|
|
|
|
| Fat (g/kg/day) |
|
|
|
|
|
|
|
| Electrolytes | |||||||
| Sodium (mmol/kg/day) |
|
|
|
|
|
|
|
| Potassium (mmol/kg/day) |
|
|
|
|
|
|
|
| Calcium (mmol/kg/day) |
|
|
|
|
|
|
|
| Magnesium (mmol/kg/day) |
|
|
|
|
|
|
|
| Phosphate (mmol/kg/day) |
|
|
|
|
|
|
|
| Micronutrient | |||||||
| Water soluble vitamins (ml/day) |
|
|
|
|
|
|
|
| Fat soluble vitamins (ml/day) | |||||||
| Trace elements (ml/day) |
|
|
|
|
|
|
|
| Total Volume (ml/kg/day) |
|
|
|
|
|
|
|
| Total Calorie (kcal/kg/day) |
|
|
|
|
|
|
|
| Duration of PN infusion (hour) |
|
|
|
|
|
|
|
| Type of PN bag |
|
|
|||||
In December 2019, following a series of discussions, the family agreed to the transition from 2-in-1 formulation to AMCB-PN regimen. Patient’s clinical progress are summarized in Table 2. Initially, when home parenteral nutrition began in 2009, the infusion duration was set at 18 hours. As the patient began attending school at the age of six, the infusion duration was gradually reduced to 12 hours, which normally started around 6.00 pm and finish at 6.00am.
| Table 2. Summary of patient clinical progress. | ||
|---|---|---|
| Date | Event | |
| Sept 2008 |
 |
Born at Hospital Tunku Jaafar, birth weight 3.6 kg. |
| Jan 2009 | Referred to Hospital Kajang due to vomiting, poor weight gain (4 kg), | |
| Transferred to Hospital Tunku Azizah (HKL) for further evaluation. | ||
| Jan 2009 | IEM screening – Normal | |
| Rectal biopsy – Ganglionic | ||
| Laparotomy, ileostomy, small bowel biopsy performed | ||
| Started on 2-in-1 PN via PICC | ||
| Mar 2009 | Bishop-Koop procedure and CVL insertion | |
| HPE: No significant inflammation | ||
| Sept 2009 | Discharged with Home PN (3 times per week regimen) | |
| Sept 2012 | Trial of stopping PN, increased oral feeding. Not successful. Continue same PN regime | |
| Dec 2019 | Transitioned from 2-in-1 PN to adult-designed commercial multichamber parenteral nutrition bags | |
Discussion
Parenteral nutrition (PN) is a vital, life-saving therapy, particularly for patients with type II and type III intestinal failure (IF) who require long-term dependency. Despite its benefits, PN is associated with high costs and significant risks, such as line infections, electrolyte imbalances, liver dysfunction, and cardiac failure.7 To address these challenges and reduce misuse, expert organizations like the British Association for Parenteral and Enteral Nutrition (BAPEN) and NICE strongly recommend that PN patients be managed by multidisciplinary Nutrition Therapy Teams (NTTs).8 These teams have been shown to improve patient outcomes and achieve cost savings.9,10 At our institution, we are proud to have established NTTs composed of doctors and dietitians from Hospital Tunku Azizah (HTA) and pharmacists from Hospital Kuala Lumpur (HKL). Pharmacists oversee the drafting, preparation, and monitoring of PN, while dietitians focus on managing enteral nutrition. Additionally, our NTTs regularly review all active in-ward and home PN patients through a minimum of three-monthly meetings, ensuring thorough oversight and tailored care. This collaborative approach minimizes risks, enhances patient outcomes, and upholds the highest standards of efficiency and quality care.
At our facility, production is conducted within a horizontal laminar flow cabinet housed in a controlled Grade B environment compliant with GMP (Good Manufacturing Practice) standards. Our in-house 2-in-1 parenteral nutrition (PN) system combines amino acids, dextrose, and electrolytes into a single infusion bag, while lipids are administered separately via a Y-site connector using 50 ml syringes. This method requires two infusion pumps and extensive line manipulation, increasing the risk of infection and infusion errors.11
In contrast, the 3-in-1 PN system combines all components—amino acids, dextrose, electrolytes, and lipids—into a single-chamber intravenous solution bag. Manual preparation involves extracting specific volumes from bulk bottles and mixing them in the Intravenous bag, making it prone to compounding errors and formulation instability due to complex chemical interactions within a single container. Although it simplifies PN administration, the 3-in-1 system poses risks related to decreased stability of the final formulation.12
Our facility lacks the equipment needed to test and maintain the specifications outlined in USP, limiting our ability to ensure long-term product stability and patient safety.13 To address this, our team uses proprietary stability-checking software from a reputable provider to identify potential incompatibilities. We limit the beyond-use date of in-house compounded PN to two days and transition to AMCB-PN whenever appropriate.
Adult-designed commercial multichambered parenteral nutrition bags (AMCB-PN) is preferred for home PN (HPN) due to its established stability, allowing convenient weekly outpatient dispensing. In our setting, all additives are incorporated under controlled conditions prior to patient use, ensuring consistency and safety. In contrast, in-house compounded PN remains viable for only a few days, requiring more frequent pharmacy visits for HPN patients.
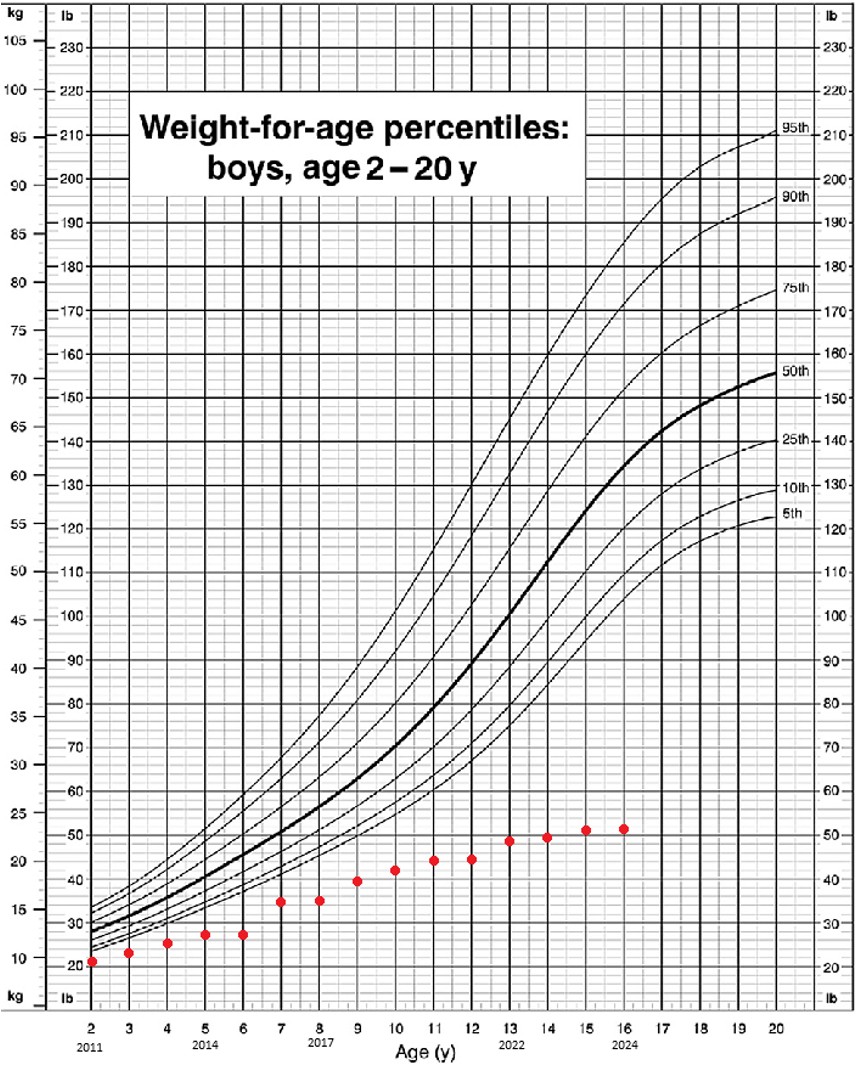
The patient’s treatment progress has been consistently monitored by healthcare professionals in collaboration with the family over the years. Currently, the patient’s body weight is recorded at range of 25.2 kg to 25.5 kg, which is below the expected weight for his age according to the Malaysian standard growth chart for boys aged 2 to 20 years.14 However, despite this, he is demonstrating positive weight gain with the use of an AMCB-PN, as indicated by data collected from 2019 to 2024 as shown in Figure 1. Although he is physically smaller than other children of the same age, he remains active and has not experienced any other medical issues.
This patient is receiving 45% to 85%, as per tabulated in Table 3, of their calorie intake through parenteral nutrition, while also benefiting from oral nutrition. He can tolerate oral intake effectively, primarily consisting of three main meals daily. This combined strategy successfully promotes his growth even in the presence of bowel dysmotility, ultimately contributing to an improved survival rate.15
| Table 3. Summary of nutrient required and delivered to patient via parenteral route. | ||||||||||
| Macro & Micronutrients |
|
|
|
|
|
|||||
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
| Energy (kcal/kg/day) |
|
|
|
|
|
|
|
|
|
|
| Amino Acids (g/kg/day) |
|
|
|
|
|
|
|
|
|
|
| Glucose (g/kg/day) |
|
|
|
|
|
|
|
|
|
|
| Lipids (g/kg/day) |
|
|
|
|
|
|
|
|
|
|
| Dextrose to Lipid Ratio |
|
|
|
|
|
|
|
|
|
|
As the lipid volume increase, there were more syringes to be infuse. Switching to an AMCB-PN significantly eliminated the need for frequent overnight syringe changes, reducing it from 5 to 6 times per night to none. This improvement greatly enhancing sleep quality for both patients and caregivers.
Utilizing an AMCB-PN minimizes interruptions during lipid syringe changes with 2-in-1 preparation, which significantly lowers the risk of infection and ensures a more continuous infusion flow. This practice not only enhances safety for patients but also contributes to improved overall outcomes by promoting consistent PN delivery without the complications associated with frequent handling and connections such as catheter related infection.16
Moreover, transitioning to an AMCB-PN system reduces the requirement from two infusion pumps to one. A single infusion pump costs approximately 3000 Malaysian Ringgit (MYR) with an annual maintenance cost of MYR 300. This change significantly reduced pump-related maintenance expenses, contributing to improved cost-effectiveness in patient care without compromising treatment protocols. Additionally, consumables such as extra 0.2-micron filters and dressing set required for 2-in-1 PN administration cost around MYR 30 per day. Eliminating the need for these consumables further reduced the overall cost. Collectively, this transition resulted in an estimated 52% reduction in total annual costs.
Although the use of commercially premixed standardized parenteral nutrition is a common practice in well-developed regions such as Europe and Australia, its adoption in developing countries like Malaysia remains limited due to niche market size and restricted availability. Additionally, adult-designed multichamber parenteral nutrition bags (AMCB-PNs) have been reported to be nutritionally inadequate for paediatric populations.4,17 However, the present case demonstrates that the use of AMCB-PN may be both feasible and safe in selected paediatric patients.
Conclusion
The case shows the shift from in-house 2-in-1 PN compounding to adult-designed commercial multichambered parenteral nutrition bags (AMCB-PN) was associated with improved ease of administration, fewer line manipulations, and reduced caregiver demands. The patient experienced enhanced sleep quality and demonstrated stable weight gain, maintaining an active lifestyle despite underlying growth limitations. Additionally, the approach helped lower equipment and consumable use, with estimated cost savings of approximately MYR 6,000 per year. These observations may offer insight into the clinical and operational benefits of AMCB-PN in selected cases.
Acknowledgement
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Ethical approval
This study has been approved by the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia (18.07.2025, RSCH ID-25-04700-Q9L). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Jochum F, Moltu SJ, Senterre T, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: fluid and electrolytes. Clin Nutr. 2018;37:2344-2353. https://doi.org/10.1016/j.clnu.2018.06.948
- Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:16-28. https://doi.org/10.1053/j.gastro.2005.12.002
- Riskin A, Picaud JC, Shamir R. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: standard versus individualized parenteral nutrition. Clin Nutr. 2018;37:2409-2417. https://doi.org/10.1016/j.clnu.2018.06.955
- Colomb V. Commercially premixed 3-chamber bags for pediatric parenteral nutrition are available for hospitalized children. J Nutr. 2013;143:2071-2076. https://doi.org/10.3945/jn.113.176974
- Meyer R, Timmermann M, Schulzke S, Kiss C, Sidler MA, Furlano RI. Developing and implementing all-in-one standard paediatric parenteral nutrition. Nutrients. 2013;5:2006-2018. https://doi.org/10.3390/nu5062006
- Joosten K, Embleton N, Yan W, Senterre T. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: energy. Clin Nutr. 2018;37:2309-2314. https://doi.org/10.1016/j.clnu.2018.06.944
- Austin PD, Stroud M, Gales BJ. Prescribing adult intravenous nutrition. Pharmaceutical press; 2007. https://doi.org/10.1345/aph.1K190
- Baker M, Harbottle L. Parenteral nutrition. In: Manual of dietetic practice. Wiley-Blackwell; 2014:357-364.
- Kennedy JF, Nightingale JM. Cost savings of an adult hospital nutrition support team. Nutrition. 2005;21:1127-1133. https://doi.org/10.1016/j.nut.2005.08.002
- Hvas CL, Farrer K, Donaldson E, et al. Quality and safety impact on the provision of parenteral nutrition through introduction of a nutrition support team. Eur J Clin Nutr. 2014;68:1294-1299. https://doi.org/10.1038/ejcn.2014.186
- Blackmer AB, Partipilo ML. Three-in-one parenteral nutrition in neonates and pediatric patients: risks and benefits. Nutr Clin Pract. 2015;30:337-343. https://doi.org/10.1177/0884533615580596
- Boullata JI, Mirtallo JM, Sacks GS, et al. Parenteral nutrition compatibility and stability: a comprehensive review. JPEN J Parenter Enteral Nutr. 2022;46:273-299. https://doi.org/10.1002/jpen.2306
- United States Pharmacopeia (USP). Globule size distribution in lipid injectable emulsions (Chapter 729). In: United States Pharmacopeial Convention. 2010.
- Kuczmarski RJ. CDC growth charts: United States. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2000.
- Bering J, DiBaise JK. Home parenteral and enteral nutrition. Nutrients. 2022;14:2558. https://doi.org/10.3390/nu14132558
- Crooks B, Harrison S, Millward G, et al. Catheter-related infection rates in patients receiving customized home parenteral nutrition compared with multichamber bags. JPEN J Parenter Enteral Nutr. 2022;46:254-257. https://doi.org/10.1002/jpen.2225
- Caba Porras I, Cabello Muriel A, Oya Alvarez de Morales B, Marín Pozo JF, García Aranda J, Llácer Pérez C. Assessment of standard parenteral nutrition in children. Nutr Hosp. 2010;25:449-455.
Copyright and license
Copyright © 2025 The author(s). This is an open-access article under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.