Abstract
Background: This is the first study to focus exclusively on a pure ischemic stroke cohort, exploring serum glutamine levels and their association with oxidative stress markers and clinical outcomes.
Methods: 50 patients diagnosed with ischemic stroke were included in our study. We used methods such as LC-MS/MS to measure glutamine levels with high accuracy. Oxidative stress markers, including total antioxidant capacity (TAC) and total oxidant status (TOS), were also analyzed. Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS), and functional outcomes were evaluated using the modified Rankin Scale (mRS) at 90 days.
Results: Serum glutamine levels varied significantly based on stroke severity. Patients with NIHSS ≥16 demonstrated significantly lower glutamine levels compared to those with NIHSS <16. Dynamic changes in glutamine levels were observed, with levels decreasing between days 1 and 4 and increasing between days 4 and 10 . Patients undergoing therapeutic interventions (tPA or endovascular therapy) showed more stable glutamine levels compared to untreated patients (P = 0.028). Elevated TOS levels independently predicted mortality (hazard ratio [HR]: 2.34, 95% CI: 1.45–3.79, P = 0.002), while persistently low glutamine levels were associated with poor outcomes (HR: 0.89, 95% CI: 0.78–0.98, P = 0.03).
Conclusion: The observed fluctuations in glutamine levels provide valuable insights into stroke severity, recovery, and mortality, suggesting their potential role in clinical prognosis. Our findings suggest that glutamine could play a critical role as a biomarker and therapeutic target in ischemic stroke, particularly in patients with NIHSS> 16.
Keywords: serum glutamine, ischemic stroke, biomarkers, clinical outcomes, oxidative stress, LC-MS/MS
Main Points
- Serum glutamine levels serve as an independent biomarker for both stroke severity and prognosis in acute ischemic stroke.
- Early reductions in glutamine levels are associated with increased oxidative stress and poor clinical outcomes.
- Patients treated with IV-tPA or endovascular therapy exhibited more stable glutamine levels, indicating the metabolic benefits of these interventions.
- Monitoring glutamine levels may guide personalized treatment strategies and support future research into glutamine-focused therapeutic approaches.
Introduction
Stroke is the second leading cause of death and the third leading cause of disability worldwide, posing a significant public health problem with profound socioeconomic consequences.1 Among all strokes, ischemic stroke accounts for 87% of cases and is characterized by acute neuronal injury, inflammation, and oxidative stress.2 Despite significant advances in acute stroke care, identifying biomarkers that predict clinical outcomes and guide therapeutic interventions remains critical.
Glutamine, the most abundant amino acid in the human body, plays a central role in metabolic and immune processes, including serving as a precursor for glutathione, glutathione regulates oxidative stress and maintains intracellular redox.3 Balance a key antioxidant that mitigates oxidative damage during ischemia.4-6 Although glutamine is synthesized endogenously under normal conditions, its levels can deplete during hypermetabolic states such as sepsis, trauma, and severe illness, which leads to its classification as a conditionally essential amino acid.6-10 Glutamine also contributes to nitrogen balance, pH regulation, and immune cell function, making it a critical player in physiological stress responses.11-13 However, its role in ischemic stroke—particularly the dynamic changes in its levels—has been underexplored.
Oxidative stress, driven by an imbalance between oxidants and antioxidants, is a major contributor to secondary neuronal injury in ischemic stroke.14 Markers such as total oxidant status (TOS) and total antioxidant capacity (TAS) provide valuable insights into the oxidative environment and its relationship with disease severity.15,16 Numerous studies explore oxidative stress in stroke, yet little is known about how glutamine dynamics interplay with these parameters.13 This gap highlights the need for further research into how these factors influence stroke outcomes.
Previous studies, such as the Redox trial, have primarily focused on heterogeneous patient populations, measuring glutamine levels only at single time points.17 In contrast, this study exclusively examines primary and clean ischemic stroke patients, offering a homogeneous cohort. By sequentially measuring serum glutamine levels on Days 1, 4, and 10, it aims to provide a comprehensive view of glutamine’s trajectory and its relationship with oxidative stress, stroke severity, and clinical outcomes.
By longitudinally analyzing serum glutamine levels in ischemic stroke patients, this study offers new perspectives on its role as a biomarker and a possible therapeutic avenue. We hope that these findings will inspire further research and pave the way for better patient care.
Materials and Methods
Study design and patient selection
This prospective study was conducted at Dicle University Faculty of Medicine Hospital between June 2023 and November 2023. Fifty patients with a confirmed diagnosis of acute ischemic stroke were included. In the power analysis conducted prior to determining the sample size, assuming α = 0.05 and an effect size of d = 0.8 (Cohen’s large effect size), a sample size of 46 patients was determined to provide 95% power. Diagnosis was based on clinical presentation, neurological examination, and neuroimaging studies conducted within 24 hours of symptom onset. Patients were subsequently admitted to the Neurology Intensive Care Unit for further management. All patients received standard polymeric enteral nutrition products during the study period to ensure consistent nutritional treatment. In this study, the IV-tPA/EVT group consisted of patients who received either intravenous tissue plasminogen activator (IV-tPA) or mechanical intervention, while the Antithrombotic Therapy Only group included those managed conservatively with antiplatelet or anticoagulant medications. Antithrombotic therapy aims to prevent clot formation and reduce the risk of recurrence in ischemic stroke. The study was approved by the Ethics Committee of Dicle University (Approval No: 149 / May 17, 2023), and informed consent was obtained from all participants or their legal representatives.
The inclusion criteria encompassed patients aged 18 years or older, admitted to the intensive care unit due to ischemic stroke, with a hospital stay of at least 10 days, and who had blood samples taken within the first 24 hours of admission. Exclusion criteria included acute or chronic kidney or liver diseases, pregnancy, use of antioxidant supplements (vitamin C or vitamin E), or treatment with glutamine-enriched nutritional solutions.
Biochemical assays
To ensure precise and sensitive measurement, serum glutamine levels were assessed at three-time points—days 1, 4, and 10—using the advanced LC-MS/MS (Liquid chromatography–mass spectrometry/mass spectrometry technique. Oxidative stress markers, including total oxidant status (TOS) and total antioxidant capacity (TAC), were measured using validated biochemical assays. The oxidative stress index (OSI) was calculated as the ratio of TOS to TAC.
Stroke severity and outcomes assessment
Stroke severity was primarily evaluated using the National Institutes of Health Stroke Scale (NIHSS) at admission, which provides a robust measure of initial stroke impact and severity. NIHSS scores were analyzed as key predictors of clinical outcomes, with higher scores indicating greater stroke severity. In patients with NIHSS scores above 16, glutamine levels were observed to decrease more significantly, further emphasizing the relationship between NIHSS and metabolic disturbances. Functional outcomes, assessed at 90 days post-stroke using the modified Rankin Scale (mRS), were used as a secondary measure to categorize recovery. Patients achieving mRS ≤ 2 were classified as having favorable outcomes, while those with mRS > 2 were classified as having poor outcomes. Mortality within 90 days was also recorded and correlated with initial NIHSS scores to highlight its prognostic significance. To minimize bias, outcome assessments were performed by blinded evaluators who were unaware of the patient’s serum glutamine and oxidative stress levels.
Statistical analysis
Descriptive statistics were used to summarize baseline demographic and clinical data. Continuous variables were presented as means ± standard deviations or medians with interquartile ranges, depending on their distribution. Categorical variables were expressed as frequencies and percentages. Group comparisons were performed using the Mann-Whitney U test or chi-square test as appropriate.
Univariate and multivariate Cox regression analyses were conducted to identify predictors of mortality and functional outcomes. Variables with a P-value < 0.1 in univariate analysis were included in multivariate models. Statistical significance was set at P < 0.05. Analyses were performed using SPSS software version 29.0 (IBM, Armonk, NY).
Results
A total of 50 ischemic stroke patients were included in the study. The median age was 65 years (range: 45–85 years), and 60% were male. The median NIHSS score at admission was 14 (range: 4–24), with 90-day mortality recorded at 20%. Baseline demographic and clinical characteristics of patients with acute ischemic stroke. The table presents data on sex distribution, median age, NIHSS scores, stroke etiology, and treatment modalities. Patients are categorized based on treatment type (IV-tPA/EVT vs. antithrombotic therapy) and 90-day mRS outcomes in Table 1.
| Table 1. Baseline demographic and clinical characteristics of the study population | ||
| Variable |
|
|
| Sex | ||
| Female |
|
|
| Male |
|
|
| Age (Median ± Range) |
|
|
| NIHSS | ||
| <16 |
|
|
| ≥16 |
|
|
| Stroke Aetiology | ||
| Cardioembolic |
|
|
| Large vessel occlusion |
|
|
| Small vessel occlusion |
|
|
| Cryptogenic |
|
|
| Other |
|
|
| Treatment | ||
| Endovascular and IV tPA Treatments |
|
|
| EVT (Endovascular Thrombectomy) |
|
|
| IV tPA (Intravenous Tissue Plasminogen Activator) |
|
|
| Antithrombotic or Anticoagulant Treatment |
|
|
| mRS at 3 Months | ||
| Fully independent (mRS < 3) |
|
|
| Dependent (3 ≤ mRS ≤ 5) |
|
|
| Mortality (mRS = 6) |
|
|
Comparative analysis of oxidative stress parameters (TAS, TOS, OSI) among survivors and non-survivors, as well as patients with NIHSS <16 and ≥16. Data are presented as mean ± standard deviation. Significant differences are highlighted, emphasizing the role of oxidative stress in stroke outcomes in Table 2.
| Data are presented as mean ± standard deviation., P values compare survivors vs. non-survivors and NIHSS <16 vs. NIHSS ≥16. TAS: Total Antioxidant Status; TOS: Total Oxidant Status; OSI: Oxidative Stress Index. | ||||||
| Table 2. Relationship between oxidative stress parameters, NIHSS, and mortality | ||||||
| Parameter |
|
|
|
|
|
|
| TAS (mmol Eq/L) |
|
|
|
|
|
|
| TOS (µmol Eq/L) |
|
|
|
|
|
|
| OSI |
|
|
|
|
|
|
Mean serum glutamine levels (µmol/L) were measured on Days 1, 4, and 10 for all patients. Temporal changes in these levels during the study period were assessed using repeated measures ANOVA, with the results detailed in Table 3, positioned below to illustrate these dynamic fluctuations and statistical comparisons comprehensively.
| Values are presented as mean ± standard deviation. P-value refers to a comparison across all three-time points, assessed using Repeated Measures ANOVA. | |||
| Table 3. Mean glutamine levels of all patients by day. | |||
| Day | Mean Glutamine Level (µmol/L) | Standard Deviation | P-value |
| Day 1 | 611.0 | 155.7 | |
| Day 4 | 600.8 | 210.6 | |
| Day 10 | 605.7 | 137.8 | 0.353 |
Serum glutamine levels varied significantly based on stroke severity. Patients with severe stroke (NIHSS ≥16) demonstrated significantly lower glutamine levels compared to those with mild-to-moderate stroke (median: 540 µmol/L vs. 620 µmol/L, P = 0.01). Oxidative stress parameters showed corresponding changes, with elevated Total Oxidant Status (TOS) and reduced Total Antioxidant Capacity (TAC) in the severe stroke group (P <0.05 for both).
Dynamic changes in serum glutamine levels during the study period were also observed. Between days 1 and 4, glutamine levels decreased by a mean of-30.0 µmol/L (standard deviation: 135.1; P = 0.124). However, an increase of 24.7 µmol/L was noted between days 4 and 10, and changes between days 1 and 10 were minimal (-5.3 µmol/L; P = 0.845).
Serum glutamine levels (µmol/L) at Days 1, 4, and 10 in patients treated with IV-tPA/EVT versus those receiving antithrombotic therapy only. The table includes within-group comparisons over time and between-group comparisons at each time point, highlighting the stabilizing effect of IV-tPA/EVT on glutamine levels in Table 4. Patients undergoing treatment (tPA or endovascular therapy) exhibited distinct patterns of serum glutamine changes compared to those not receiving treatment. Treated patients demonstrated more stable glutamine levels over the study period, while untreated patients showed a marked decline. A significant difference in glutamine changes between days 1 and 10 was observed between treated and untreated groups (29.1 µmol/L vs. -66.5 µmol/L, P = 0.028).
| Data are presented as mean ± standard deviation. P** compares values within the group across time points. P compares values between treated and untreated groups at the same time points. | |||||
| Table 4. Glutamine levels in treated and untreated patients | |||||
| Parameter |
|
|
|
|
|
| Glutamine at Day 1 (µmol/L) |
|
|
|
|
|
| Glutamine at Day 4 (µmol/L) |
|
|
|
||
| Glutamine at Day 10 (µmol/L) |
|
|
|
||
| Change (Day 1 to Day 4) |
|
|
|
|
|
| Change (Day 4 to Day 10) |
|
|
|
||
| Change (Day 1 to Day 10) |
|
|
|
||
In univariate Cox regression analysis, elevated TOS levels were associated with a higher risk of mortality (hazard ratio [HR]: 2.34, 95% CI: 1.45–3.79, P = 0.002). Serum glutamine levels independently predicted favorable 90-day functional outcomes (mRS ≤ 2, HR: 0.89, 95% CI: 0.78–0.98, P = 0.03). Multivariate analysis confirmed the independent prognostic value of serum glutamine levels, along with age and NIHSS score at admission.
Additionally, serum glutamine levels on admission were found to correlate inversely with stroke severity as determined by NIHSS score. Patients with persistently low glutamine levels throughout the study period were more likely to exhibit poor outcomes, emphasizing the importance of glutamine depletion as a prognostic factor. Furthermore, the significant association between oxidative stress markers (elevated TOS and reduced TAC) and glutamine levels highlights the interplay between metabolic and oxidative pathways in ischemic stroke severity and progression.
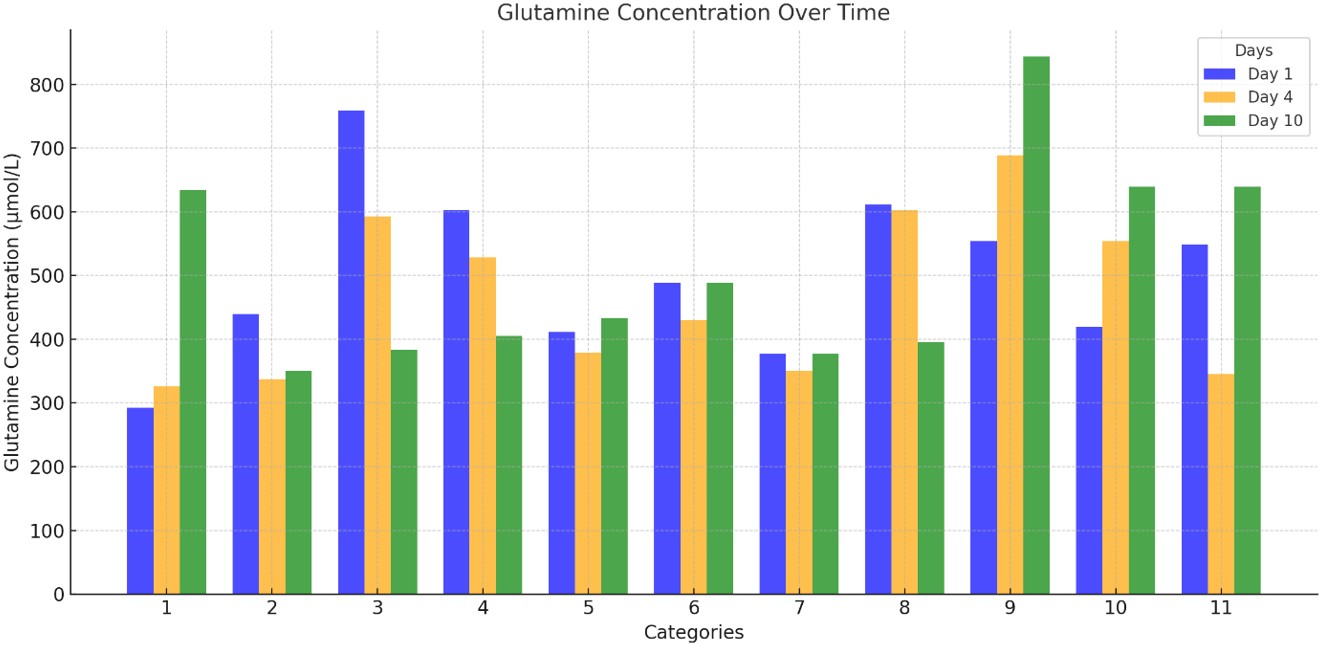
Figure 1. Glutamine concentrations (µmol/L) were measured on Days 1, 4, and 10 across different patient/follow-up categories. Day 1 (blue): The highest glutamine levels were generally observed. Day 4 (orange): A significant decline was noted in most categories, consistent with a stress response or catabolic state. Day 10 (green): Partial recovery or stabilization was observed in some categories (particularly 1, 6, 9, 10, and 11). These changes highlight time-dependent, individualized metabolic responses and inter-category heterogeneity.
Discussion
This study provides novel insights into the dynamic changes in serum glutamine levels and their association with clinical outcomes in acute ischemic stroke patients. Our findings demonstrate that lower serum glutamine levels are significantly associated with greater stroke severity and worse functional outcomes, emphasizing the potential role of glutamine as a biomarker for ischemic stroke prognosis.
Oxidative stress plays a critical role in the pathophysiology of ischemic stroke.14-16 Elevated total oxidant status (TOS) and reduced total antioxidant capacity (TAS) observed in this study align with prior evidence highlighting heightened oxidative stress in severe stroke cases. However, integrating serum glutamine levels with oxidative stress parameters offers a unique perspective on the metabolic disturbances influencing stroke severity and recovery. Glutamine, known for its role in glutathione synthesis and nitrogen balance maintenance, appears to reflect a systemic response to acute stress, consistent with findings in critically ill populations.6,11,12
The decline in glutamine levels during the early hospitalization phase observed in this study likely reflects its utilization in critical processes such as immune modulation and stress response. This trajectory supports previous studies reporting reduced glutamine levels in hypercatabolic states, including sepsis and trauma.8,11
The subsequent increase in glutamine levels after day 4, particularly among patients undergoing therapeutic interventions such as endovascular therapy or tissue plasminogen activator (tPA) administration, suggests a metabolic recovery facilitated by these treatments.17
A key finding of this study is the independent predictive value of serum glutamine levels for functional outcomes at 90 days, alongside established predictors such as age and NIHSS score. This underscores glutamine’s potential utility as a biomarker to guide clinical decisions and tailor therapeutic strategies for ischemic stroke patients.4,5 These results complement earlier studies demonstrating the prognostic significance of glutamine in critically ill patients.11,12,17-19
Employing blinded evaluators and applying standardized protocols for patient inclusion mitigated potential sources of bias. However, the single-center design may limit the generalizability of the findings.
While the findings are promising, the single-center design and relatively small sample size may limit the generalizability of the results. Multicenter studies with larger cohorts are needed to confirm these findings and enhance external validity. Moreover, the lack of longitudinal glutamine measurements beyond 10 days and the absence of a control group without stroke warrant a cautious interpretation of the results. Future research with larger, multicenter cohorts and extended follow-up periods is needed to validate these findings and further explore the mechanistic pathways linking glutamine metabolism to stroke outcomes.
In conclusion, our study highlights the prognostic significance of serum glutamine levels in acute ischemic stroke. Glutamine serves not only as a marker for stroke severity but also as a potential target for therapeutic intervention. These findings open new avenues for research into integrating glutamine-focused strategies into comprehensive stroke management protocols, addressing both the metabolic and oxidative stress components of ischemic stroke.
Conclusion
This study underscores the critical role of serum glutamine levels as a biomarker for ischemic stroke severity and prognosis. Our findings demonstrate that dynamic changes in glutamine levels during the acute phase of stroke are strongly associated with stroke severity, as measured by the NIHSS score, and functional outcomes at 90 days. The integration of glutamine measurements with oxidative stress parameters provides a novel perspective on the metabolic and physiological disturbances in ischemic stroke, highlighting new avenues for research and clinical intervention. Specifically, persistently low glutamine levels were found to correlate with poor outcomes, while treated patients exhibited more stable glutamine levels over time compared to untreated patients.
The self-controlled design of this study enabled the analysis of dynamic glutamine changes within the same patient cohort, minimizing inter-patient variability. This focus on a well-defined, primary ischemic stroke group enhances the specificity and relevance of our findings, differentiating this study from prior research involving heterogeneous or mixed patient populations.
Future studies should validate these findings in larger, multicenter cohorts and explore the mechanistic pathways linking glutamine metabolism to neuronal recovery and functional improvement. Additionally, the potential role of glutamine supplementation in improving metabolic and oxidative outcomes warrants further investigation, particularly in patients with severe stroke and persistently low glutamine levels. These efforts will enhance our understanding of stroke pathophysiology and improve patient outcomes through personalized medicine approaches.
Ethical approval
This study has been approved by the the Ethics Committee of Dicle University (approval date 17.05.2023, number 149). Written informed consent was obtained from the participants.
Source of funding
Nestle provided biochemical kit support for the analysis of glutamine, total antioxidant status (TAS), and total oxidant status (TOS).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17:18-29. https://doi.org/10.1177/17474930211065917
- Go S, Worman D. Stroke, transient ischemic attack, and cervical artery dissection. In: Tintinalli JE, editor. Emergency medicine: a comprehensive study guide. 7th ed. New York: McGraw-Hill; 2010:1122-1135.
- Sen CK. Nutritional biochemistry of cellular glutathione. J Nutr Biochem. 1997;8:660-672. https://doi.org/10.1016/S0955-2863(97)00113-7
- Cengiz M, Borku Uysal B, Ikitimur H, et al. Effect of oral l-Glutamine supplementation on Covid-19 treatment. Clin Nutr Exp. 2020;33:24-31. https://doi.org/10.1016/j.yclnex.2020.07.003
- Santos HO, Tinsley GM, da Silva GAR, Bueno AA. Pharmaconutrition in the clinical management of COVID-19: a lack of evidence-based research but clues to personalized prescription. J Pers Med. 2020;10:145. https://doi.org/10.3390/jpm10040145
- Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619-634. https://doi.org/10.1038/nrc.2016.71
- Cruzat VF, Pantaleão LC, Donato J, de Bittencourt PI, Tirapegui J. Oral supplementations with free and dipeptide forms of L-glutamine in endotoxemic mice: effects on muscle glutamine-glutathione axis and heat shock proteins. J Nutr Biochem. 2014;25:345-352. https://doi.org/10.1016/j.jnutbio.2013.11.009
- Kao C, Hsu J, Bandi V, Jahoor F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am J Physiol Endocrinol Metab. 2013;304:E1359-E1364. https://doi.org/10.1152/ajpendo.00628.2012
- Rogero MM, Borges MC, Pires ISO, Borelli P, Tirapegui J. Effect of glutamine supplementation and in vivo infection with Mycobacterium bovis (Bacillus Calmette-Guérin) in the function of peritoneal macrophages in early-weaned mice. Ann Nutr Metab. 2007;51(Suppl. 1):173-174. https://doi.org/10.1159/000105120
- Mortada H, Alhindi N, Abukhudair A, Alanazi S, AlSahli A, Arab K. The Effects of glutamine supplementation on reducing mortality and morbidity among burn patients: a systematic review and meta-analysis of randomized controlled trials. JPRAS Open. 2022;35:6-17. https://doi.org/10.1016/j.jpra.2022.09.003
- Karinch AM, Pan M, Lin CM, Strange R, Souba WW. Glutamine metabolism in sepsis and infection. J Nutr. 2001;131: 2535S-2538S. https://doi.org/10.1093/jn/131.9.2535S
- Rodas PC, Rooyackers O, Hebert C, Norberg Å, Wernerman J. Glutamine and glutathione at ICU admission in relation to outcome. Clin Sci (Lond). 2012;122:591-597. https://doi.org/10.1042/CS20110520
- Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate-their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1-9. https://doi.org/10.1002/cbf.1003
- Leinonen JS, Ahonen JP, Lönnrot K, et al. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31:33-39. https://doi.org/10.1161/01.str.31.1.33
- Menon B, Ramalingam K, Kumar R. Evaluating the role of oxidative stress in acute ischemic stroke. J Neurosci Rural Pract. 2020;11:156-159. https://doi.org/10.1055/s-0039-3402675
- Karaaslan F, Demir F, Yılmaz R, Akıl E. Total oxidant/antioxidant status, copper and zinc levels in acute ischemic stroke patients after mechanical thrombectomy. Clin Neurol Neurosurg. 2023;229:107718. https://doi.org/10.1016/j.clineuro.2023.107718
- Heyland DK, Dhaliwal R. Role of glutamine supplementation in critical illness given the results of the REDOXS study. JPEN J Parenter Enteral Nutr. 2013;37:442-443. https://doi.org/10.1177/0148607113488421
- van der Hulst RR, van Kreel BK, von Meyenfeldt MF, et al. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363-1365. https://doi.org/10.1016/0140-6736(93)90939-e
- Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128:1249-1252. https://doi.org/10.1093/jn/128.8.1249
Copyright and license
Copyright © 2025 The author(s). This is an open-access article under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.