Abstract
Objective: Enteral nutrition (EN) is a cornerstone of nutritional support for patients unable to meet their dietary needs orally, yet concerns remain regarding the adequacy of micronutrient provision in commercial enteral formulas. The aim of the study is to evaluate the micronutrient content of commonly used adult enteral formulas in Turkiye by comparing their composition with the recommendations provided in international and national guidelines.
Methods: Thirty-eight commercially available adult enteral formulas, encompassing standard, immune-modulating, and disease-specific types were analyzed. The micronutrient content was calculated based on the labelled values and subsequently adjusted for daily energy intakes of 1500 and 1800 kcal. These values were then compared with Dietary Reference Intakes (DRIs), ESPEN micronutrient guidelines, and TUBER-2022 recommendations.
Results: At a 1500 kcal/day EN intake, 89.1% of the formulas (33/37) failed to meet the recommended intake for vitamin K, 86.5% (32/37) for vitamin D, and 94.6% (35/37) for magnesium based on DRI and ESPEN guidelines. Additionally, 37.8% (14/37) of the formulas did not meet the iron requirement specifically for females according to DRI and ESPEN recommendations. According to ESPEN’s recommendations for high demand, all formulas were found to be insufficient in multiple micronutrients, including vitamins A, D, E and B-complex vitamins, as well as iron, zinc, selenium, chromium and molybdenum. Furthermore, one immune-modulating formula exceeded the tolerable upper intake levels for five micronutrients (folic acid, calcium, magnesium, zinc, and manganese) according to both the DRI and the TUBER-2022.
Conclusion: Commonly used enteral formulas in Turkey may inadequately supply essential micronutrients, particularly to vulnerable populations with increased requirements. These findings emphasize the need for routine clinical monitoring, individualized supplementation strategies and reformulation of products, especially with regard to vitamin D and magnesium content, to align with ESPEN’s higher intake recommendations.
Keywords: enteral nutrition, micronutrient, DRI, ESPEN, TUBER
Main Points
- The majority of adult enteral formulas available in Turkey do not meet the recommended intake levels for key micronutrients, particularly vitamin D, vitamin K, magnesium, and iron.
- In comparison with the higher intake recommendations set out by ESPEN, a more extensive insufficiency of micronutrients was identified.
- These findings emphasise the necessity of revising product formulations and strengthening clinical strategies to align with international and national nutritional standards.
Introduction
Enteral nutrition (EN) is a key part of nutritional management for patients who cannot meet their dietary requirements through oral intake, but who have an intact and functional gastrointestinal tract. EN plays a critical role in preserving gut integrity, supporting immune function and mitigating the risks of malnutrition in various clinical contexts.1 The increasing variety and range of commercial enteral formulas provide clinicians with options tailored to specific patient needs, including standard, immune-modulating and disease-specific formulations.2
Enteral formulas are typically developed based on the Dietary Reference Intakes (DRIs). They are designed to deliver essential nutrients—including macronutrients, micronutrients, and antioxidants—to meet the daily nutritional needs of diverse patient populations, ranging from critically ill, hypermetabolic individuals to stable patients receiving long-term home enteral nutrition.3 However, DRIs primarily represent reference intake values intended for healthy individuals and general population groups.4 Despite the widespread use of enteral formulas, concerns persist regarding whether their micronutrient content adequately meets the nutritional demands outlined by current clinical guidelines.
Micronutrients, including vitamins, minerals and trace elements, are essential for many physiological processes, including immune defense, enzymatic activity and metabolism. Both micronutrient deficiencies and excesses may adversely affect clinical outcomes, particularly in vulnerable populations such as older adults, individuals with chronic diseases.5,6 Patients receiving prolonged enteral nutrition are particularly susceptible to micronutrient imbalances, which can result in significant clinical complications, including deficiencies and toxicities.7-9
To ensure optimal patient care, it is essential to systematically assess the micronutrient profiles of commercially available enteral formulas by comparing them with established international and national dietary guidelines.10 Key reference standards include the Dietary Reference Intakes (DRIs)11, the micronutrient guideline of the European Society for Clinical Nutrition and Metabolism (ESPEN)5, and the Turkish Nutrition Guideline (TUBER-2022).12 The DRIs11 provide nutrient intake recommendations primarily based on healthy populations and serve as a general reference for adequate nutrient consumption. In contrast, ESPEN guideline5 focus specifically on clinical nutrition, offering tailored recommendations for patients with diverse medical conditions and metabolic demands. TUBER-202212, as a national guideline, integrates local nutritional considerations and population-specific data to guide clinical nutrition practices in Türkiye. These standards provide recommendations for appropriate micronutrient intake in clinical nutrition practice.
In Türkiye, the use of enteral nutrition formulas is steadily increasing; however, there is limited data available regarding their micronutrient adequacy compared to international and national guidelines. This study aims to evaluate the micronutrient contents of commonly used adult enteral formulas in Türkiye and compare them with the recommendations of DRI, ESPEN, and TUBER-2022, thereby providing clinicians with essential information to guide formula selection and optimize nutritional management.
Methods
This study evaluated 38 adult EN formulas produced by three different manufacturers and available commercially in Türkiye. The formulas were categorized into three groups: 21 standard EN formulas, six immune-modulating formulas, and 11 disease-specific formulas (for diabetes, pulmonary disease, renal disease, and malabsorption). Nutritional data were extracted from product labels available on official websites or technical documents provided by manufacturers.
In this study, nutrient reference values were obtained from several authoritative sources to ensure comprehensive assessment. The DRI11 provided Recommended Dietary Allowance (RDA), which represents the average daily intake sufficient to meet the nutrient requirements of nearly all healthy individuals, and Tolerable Upper Intake Level (UL), indicating the maximum daily intake unlikely to cause adverse health effects. The ESPEN5 supplied minimum and maximum nutrient requirements, defining safe intake ranges applicable in clinical nutrition settings. Additionally, the TUBER-202212 offered Adequate Intake (AI), recommended when RDA values are not established, along with UL values to identify upper intake limits.
The micronutrient content of each product was calculated based on the labelled values and standardized to daily energy intakes of 1500 and 1800 kcal/day.10,13 These values were then compared with the recommendations of the DRIs11, ESPEN micronutrient guideline5, and the TUBER-2022.12 The average micronutrient levels were then assessed, and nutrients that did not meet the recommended levels were examined in detail across all enteral formulas.
Data analysis was performed using RStudio software (version 2022.07.1, RStudio PBC, Boston, MA, USA). Continuous variables were presented as median (minimum–maximum). Micronutrients that did not meet the recommended intake thresholds were identified and analyzed in more detail across formula categories. This study was designed as a descriptive analysis aiming to compare micronutrient contents with established guidelines; therefore, no statistical hypothesis testing was conducted.
Results
The micronutrient contents of the evaluated enteral formulas at a 1500 kcal intake, along with the corresponding reference values and tolerable upper intake levels based on DRI, ESPEN, and TUBER-2022 guidelines, are summarized in Table 1. Additionally, Figure 1 illustrates the percentages of micronutrient provision relative to the recommended daily intake levels.
| AI: Adequate intake, M: male, f: female, UL: tolerable upper intake levels., DRI: Dietary Reference Intakes, ESPEN: European Society for Clinical Nutrition and Metabolism, TUBER: Türkiye Nutrition Guideline. | ||||||||||
| Table 1. Micronutrient Content of Enteral Formulas at 1500 kcal/day Compared to Recommendations from DRIs, ESPEN Guidelines, and TUBER-2022. | ||||||||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|||||
| Vitamin A (mcg) |
|
|
|
|
|
|
|
|
|
|
| Vitamin D (mcg) |
|
|
|
|
|
|
|
|
|
|
| Vitamin E (mg) |
|
|
|
|
|
|
|
|
|
|
| Vitamin K (µg) |
|
|
|
|
|
|
|
|
|
|
| Thiamine (mg) |
|
|
|
|
|
|
|
|
|
|
| Riboflavin (mg) |
|
|
|
|
|
|
|
|
|
|
| Niacin (mg) |
|
|
|
|
|
|
|
|
|
|
| Pantothenic acid (mg) |
|
|
|
|
|
|
|
|
|
|
| Vitamin B6 (mg) |
|
|
|
|
|
|
|
|
|
|
| Biotin (µg) |
|
|
|
|
|
|
|
|
|
|
| Folic acid (µg) |
|
|
|
|
|
|
|
|
|
|
| Vitamin B12 (µg) |
|
|
|
|
|
|
|
|
|
|
| Vitamin C (mg) |
|
|
|
|
|
|
|
|
|
|
| Sodium (mg) |
|
|
|
|
|
|
|
|
|
|
| Potassium (mg) |
|
|
|
|
|
|
|
|
|
|
| Chloride (mg) |
|
|
|
|
|
|
|
|
|
|
| Calcium (mg) |
|
|
|
|
|
|
|
|
|
|
| Phosphorus (mg) |
|
|
|
|
|
|
|
|
|
|
| Magnesium (mg) |
|
|
|
|
|
|
|
|
|
|
| Iron (mg) |
|
|
|
|
|
|
|
|
|
|
| Zinc (mg) |
|
|
|
|
|
|
|
|
|
|
| Copper (µg) |
|
|
|
|
|
|
|
|
|
|
| Iodine (µg) |
|
|
|
|
|
|
|
|
|
|
| Selenium (µg) |
|
|
|
|
|
|
|
|
|
|
| Chromium (µg) |
|
|
|
|
|
|
|
|
|
|
| Molybdenum (µg) |
|
|
|
|
|
|
|
|
|
|
| Manganese (mg) |
|
|
|
|
|
|
|
|
|
|
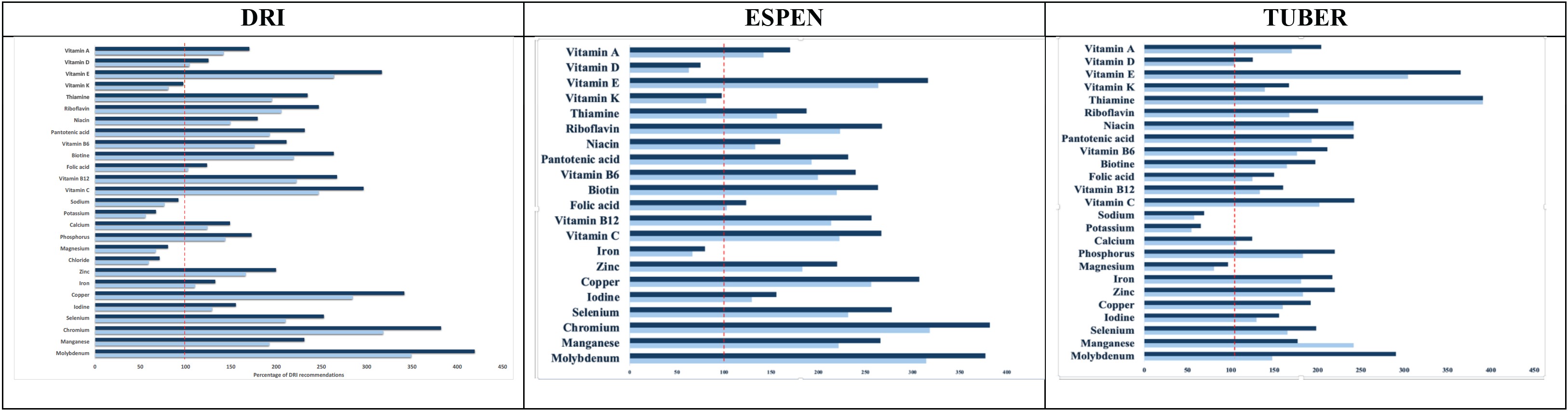
According to the DRI, the enteral formulas provided 56.10–67.32% of the recommended daily potassium intake, 59.46–71.36% of chloride, 67.17–80.61% of magnesium, 76.79–92.14% of sodium, and 81.20–97.43% of vitamin K when administered at energy levels of 1500-1800 kcal per day. Eleven other micronutrients met or exceeded daily requirements and remained well below the established tolerable upper intake levels, indicating a safe intake range for these nutrients.
Based on ESPEN guidelines, the formulas provided 62.58–75.10% of the recommended daily vitamin D intake, 66.39–79.66% of the recommended daily iron intake, and 81.20–97.44% of the recommended daily vitamin K intake, all at the same energy levels. Other micronutrients were supplied in adequate amounts.
When evaluated in reference to the TUBER-2022 guidelines, the formulas provided 54.50–65.40% of the recommended potassium intake and 57.59–69.11% of the recommended sodium intake. Other micronutrients met or exceeded daily requirements while remaining safely below the upper intake limits, suggesting no potential risk of excessive intake.
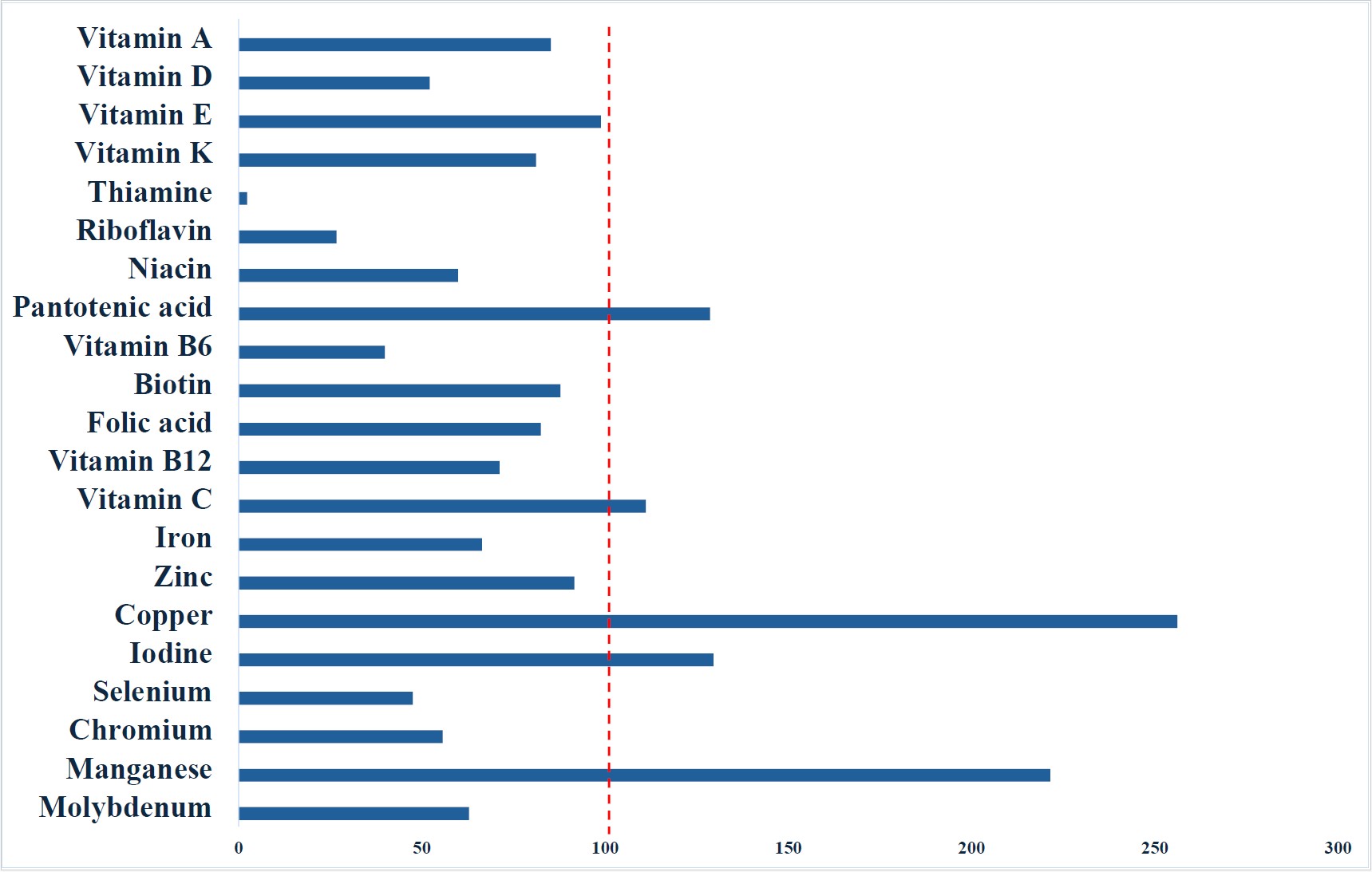
However, when evaluated according to the higher intake recommendations outlined in ESPEN guidelines, the enteral formulas were insufficient in providing several key micronutrients at a 1500 kcal/day intake. These included vitamins A, D, and E, B-complex vitamins (thiamine, riboflavin, niacin, vitamin B6, biotin, folic acid, and vitamin B12), as well as the minerals iron, zinc, selenium, chromium, and molybdenum (Figure 2).
Detailed evaluation of vitamin D, vitamin K, iron, and magnesium contents at both 1500 kcal and 1800 kcal intake levels revealed persistent inadequacies across most formulas, as presented in Figure 3. With the exception of one immune-modulating formula, the remaining products failed to provide sufficient vitamin D intake according to ESPEN recommendations at 1500 kcal/day. Additionally, based on DRI and TUBER-2022 criteria, 45.9% (17 out of 37) of the enteral formulas were inadequate in meeting the daily vitamin D requirement at this energy level.
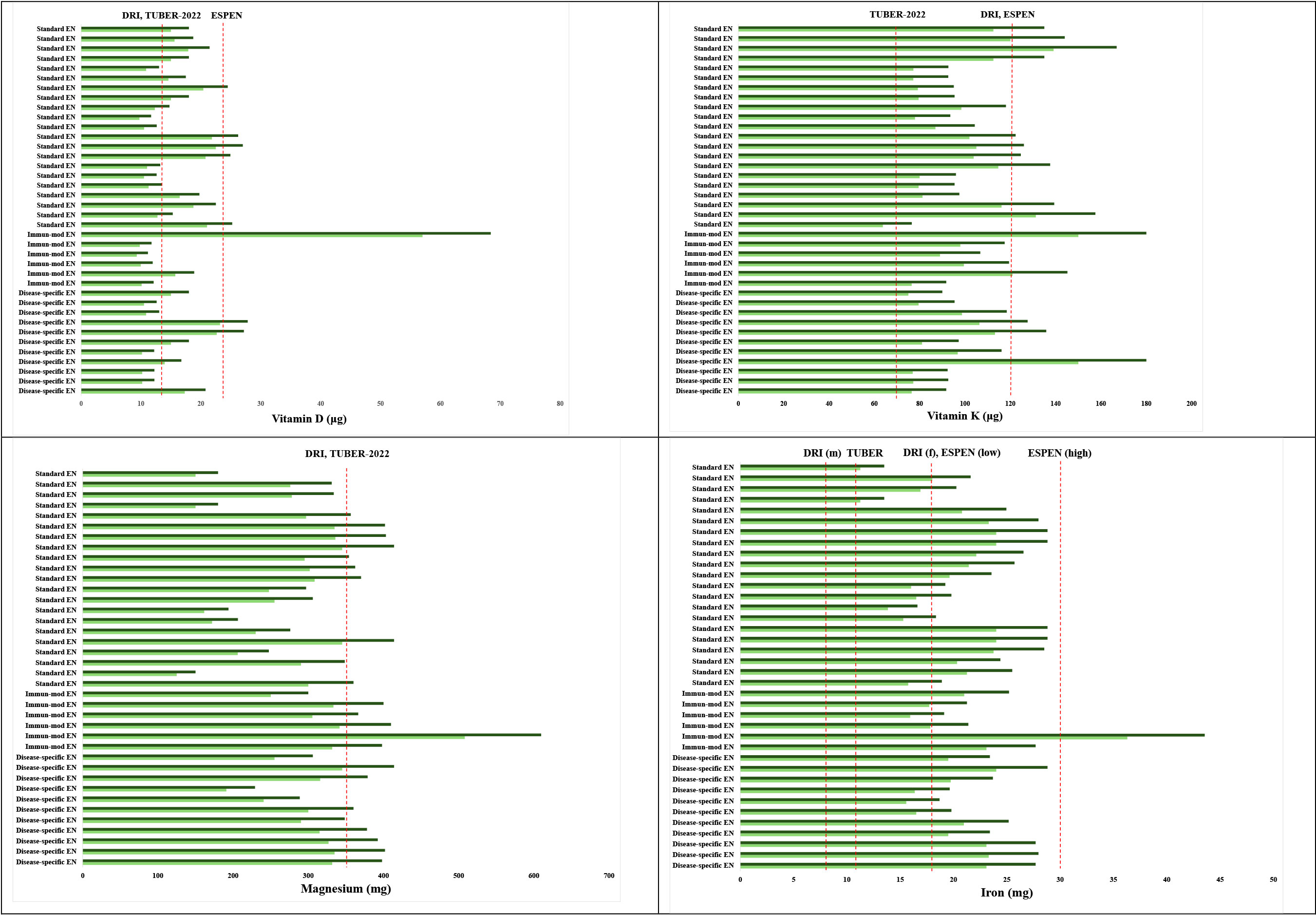
Although 81.1% (30 out of 37) of the formulas met the vitamin K requirement according to TUBER-2022 guidelines, adequacy rates significantly declined when compared to the standards of DRI and ESPEN, with only 10.8% (4 out of 37) meeting the recommended levels. In contrast, 89.1% (33 out of 37) of the formulas failed to meet the vitamin K requirements according to DRI and ESPEN guidelines (Figure 3).
Regarding magnesium, only one immune-modulating formula (2.6%) met the minimum requirements defined by both DRI and TUBER-2022 at an intake of 1500 kcal/day.
All enteral formulas provided sufficient iron at 1500 kcal/day for males based on both DRI and TUBER-2022 recommendations. However, 37.8% (14 out of 37) of the formulas did not meet the iron requirement for females according to DRI and the lower reference range of ESPEN. Furthermore, one formula achieved the higher iron intake recommended by ESPEN for this energy level (Figure 3).
Finally, among the 38 evaluated formulas, one immune-modulating formula exceeded the UL for five micronutrients (folic acid, calcium, magnesium, zinc, and manganese) according to both DRI and TUBER-2022 guidelines.
Discussion
To the best of our knowledge, this is the first study to investigate the micronutrient content of enteral formulas available in Türkiye. The study demonstrated that the intake of vitamin D, vitamin K, magnesium, iron, sodium, potassium, and chloride provided by a 1500 kcal/day enteral formula is insufficient when compared to established reference values. Among these micronutrients, vitamin D, vitamin K, magnesium, and iron were particularly inadequate in most formulas, as assessed according to the DRI, ESPEN, and TUBER-2022 guidelines, with variations depending on formula type and gender-specific requirements. Additionally, when the higher intake recommendations proposed by ESPEN were considered, inadequacies extended to a broader range of micronutrients, including vitamin A, vitamin E, B-complex vitamins, zinc, selenium, chromium, and molybdenum.
Current guideline emphasize that micronutrient intake is directly influenced by daily energy intake.5 Existing literature has predominantly focused on the micronutrient intake of hospitalized patients, particularly those in intensive care unit (ICU). A systematic review including nine studies assessing micronutrient intake in ICU patients reported that vitamin B12, vitamin D, vitamin C, vitamin A, thiamine, iron, folate, zinc, and selenium were adequately provided by enteral feeding volumes averaging 826–1600 mL/day, in accordance with the DRIs (7). It is important to note that DRIs typically offer more conservative recommendations tailored to healthy or apparently healthy populations. In contrast, ESPEN guidelines encompass more comprehensive micronutrient recommendations that specifically address the needs of critically ill patients, which may explain discrepancies observed between the two frameworks.
Despite the widespread use of enteral nutrition, studies evaluating micronutrient intake in comparison with ESPEN guidelines remain limited. A study conducted among 81 ICU patients demonstrated that an average enteral nutrition intake of 1037 kcal/day was insufficient to meet ESPEN recommendations for several micronutrients, including vitamins A, D, C, E, as well as selenium, manganese, and zinc.14 Similarly, a previous study involving 226 ICU patients demonstrated that even when enteral nutrition intake exceeded 1500 kcal/day, the provision of niacin and vitamin D remained insufficient when evaluated in accordance with standard ESPEN guidelines.15
Nevertheless, when assessing micronutrient intake, it is crucial to evaluate the actual micronutrient amounts contained in enteral formulas. An evaluation of formula composition reveals that micronutrient inadequacies persist even at commonly recommended energy intakes of 1500–1800 kcal/day. Supporting our findings, Yang et al.10 investigated the micronutrient content of 31 widely used commercial enteral formulas in China and similarly reported that vitamin D, vitamin K, and iron intakes at 1500 kcal/day were inadequate according to ESPEN guidelines, despite being sufficient according to the Chinese DRI standards. Likewise, another study evaluating 62 widely used commercial enteral formulas in relation to dietary reference values for European and Italian populations found that at a 1500 kcal/day intake, these formulas provided insufficient amounts of vitamin K and fluoride, while some exceeded the tolerable upper intake levels for zinc and vitamin A.13 These results collectively indicate that enteral formulas may not consistently ensure adequate micronutrient provision unless caloric intake exceeds specific thresholds. Therefore, the potential need for additional micronutrient supplementation, particularly in patients on long-term enteral nutrition, should be carefully considered.
Patients receiving long-term EN are at increased risk of adverse clinical outcomes due to potential micronutrient deficiencies.9 Our findings indicate that EN formulas may inadequately supply vitamin D, vitamin K, magnesium, and iron. Vitamin D, vitamin K, and magnesium are essential for maintaining bone health, muscle function, and immune regulation.5,16 Deficiencies in these micronutrients have been linked to complications such as impaired immune responses, increased risk of infections, and susceptibility to falls and fractures, although the current study did not assess clinical outcomes at the patient level.17-19 Additionally, the iron content of the evaluated enteral formulas was notably lower than the reference values, particularly for women of reproductive age. Inadequate iron intake has been associated in the literature with various adverse clinical outcomes, including increased risks of cardiovascular disease, diabetes, certain cancers (such as breast and colorectal cancer), and depression. However, these potential associations were not directly evaluated in this study.20
It is particularly important to consider the high-requirement recommendations outlined by ESPEN for critically ill patients and individuals with acute malnutrition. These recommendations are intended for short-term repletion (typically not exceeding 15 days) to avoid the need for intravenous micronutrient supplementation.5 However, our findings indicate that most of the evaluated enteral formulas fall considerably short of meeting these elevated requirements. This insufficient intake may increase the risk of adverse clinical outcomes, highlighting the importance of careful monitoring, consideration of appropriate supplementation, and possibly reformulation of existing products to better meet clinical needs. Further research assessing patient-level outcomes is warranted.
Furthermore, our data indicated inadequate sodium, potassium, and chloride content in enteral formulas. However, these electrolytes are naturally present in drinking water, and patients receiving enteral nutrition may meet their requirements through additional water intake. Moreover, we did not identify any data indicating adverse clinical outcomes specifically associated with deficiencies of these electrolytes under these conditions.
This study has several limitations that should be acknowledged. First, the micronutrient data were derived from manufacturers’ product labels rather than direct laboratory analyses; thus, potential discrepancies between declared and actual nutrient content cannot be ruled out. Second, the analysis was limited to formulas available in the Turkish market, which may limit the generalizability of findings to other countries or regions. Third, only the theoretical nutrient content based on standardized daily energy intakes (1500 and 1800 kcal/day) was evaluated; actual patient intake may vary due to interruptions in feeding, gastrointestinal tolerance, or clinical conditions. Additionally, this study did not assess the bioavailability or clinical outcomes associated with micronutrient intake, which may further influence nutritional adequacy. Finally, no statistical comparisons or hypothesis testing were performed, as the primary aim of the study was to provide a descriptive evaluation and guideline-based comparison. Additionally, the relatively small sample sizes within each formula subgroup limited the feasibility of meaningful statistical analysis.
In conclusion, this study highlights that adult enteral formulas commonly used in clinical practice may not sufficiently meet the recommended intake levels of several essential micronutrients, particularly when assessed according to ESPEN guidelines. Micronutrient inadequacies, especially in vitamin D, vitamin K, magnesium, and iron, persist even at energy intakes of 1500–1800 kcal/day. These deficiencies may have significant clinical implications, especially for patients on long-term enteral nutrition or those with increased micronutrient requirements, such as critically ill individuals. The potential need for additional micronutrient supplementation should be carefully considered in routine clinical practice to prevent adverse health outcomes. Furthermore, the findings emphasize the importance of revisiting the formulation of enteral products to ensure they adequately address the micronutrient needs of ICU patient populations. Further research is warranted to explore the clinical impact of these inadequacies and to establish optimal supplementation strategies. Specifically, well-designed interventional studies are needed to evaluate the effectiveness and safety of micronutrient supplementation in different patient populations receiving enteral nutrition. Such studies would provide valuable evidence to guide clinical decision-making and improve patient outcomes.
Ethical approval
Ethical review and approval were waived for this study, as it exclusively analyzed product contents and did not involve experiments with humans or animals.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Boullata JI, Carrera AL, Harvey L, et al. ASPEN safe practices for enteral nutrition therapy. JPEN J Parenter Enteral Nutr. 2017;41:15-103. https://doi.org/10.1177/0148607116673053
- Church A, Zoeller S. Enteral nutrition product formulations: a review of available products and indications for use. Nutr Clin Pract. 2023;38:277-300. https://doi.org/10.1002/ncp.10960
- Doley J. Enteral Nutrition Overview. Nutrients. 2022;14:2180. https://doi.org/10.3390/nu14112180
- Chung M, Balk EM, Ip S, et al. Reporting of systematic reviews of micronutrients and health: a critical appraisal. Am J Clin Nutr. 2009;89:1099-1113. https://doi.org/10.3945/ajcn.2008.26821
- Berger MM, Shenkin A, Schweinlin A, et al. ESPEN micronutrient guideline. Clin Nutr. 2022;41:1357-1424. https://doi.org/10.1016/j.clnu.2022.02.015
- Koekkoek WA, Hettinga K, de Vries JH, van Zanten AR. Micronutrient deficiencies in critical illness. Clin Nutr. 2021;40:3780-3786. https://doi.org/10.1016/j.clnu.2021.05.003
- Breik L, Tatucu-Babet OA, Ridley EJ. Micronutrient intake from enteral nutrition in critically ill adults: a systematic review of randomised controlled trials. Aust Crit Care. 2022;35:564-574. https://doi.org/10.1016/j.aucc.2021.09.001
- Breik L, Tatucu-Babet OA, Paul E, Duke G, Elliott A, Ridley EJ. Micronutrient intake from enteral nutrition in critically ill adult patients: a retrospective observational study. Nutrition. 2022;95:111543. https://doi.org/10.1016/j.nut.2021.111543
- Osland EJ, Polichronis K, Madkour R, Watt A, Blake C. Micronutrient deficiency risk in long-term enterally fed patients: a systematic review. Clin Nutr ESPEN. 2022;52:395-420. https://doi.org/10.1016/j.clnesp.2022.09.022
- Yang H, Hou L, Sun HM, Ye SH. Comparison of micronutrients in adult enteral formulas widely used in clinical practice. Food Sci Nutr. 2023;11:6096-6105. https://doi.org/10.1002/fsn3.3545
- National Institutes of Health. Nutrient recommendations: Dietary Reference Intakes (DRI). 2023. Available at: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx
- Republic of Türkiye, Ministry of Health, General Directorate of Public Health. Türkiye Beslenme Rehberi (TUBER) 2022. Available at: https://hsgm.saglik.gov.tr/tr/web-uygulamalarimiz/357.html
- Iacone R, Scanzano C, Alfonsi L, et al. Daily macro and micronutrient supply for patients on total enteral nutrition: are they in keeping with new dietary reference intakes for the Italian population? Nutr Metab Cardiovasc Dis. 2014;24:e15-e17. https://doi.org/10.1016/j.numecd.2013.10.023
- Kasti AN, Theodorakopoulou M, Katsas K, et al. Factors associated with interruptions of enteral nutrition and the impact on macro- and micronutrient deficits in ICU patients. Nutrients. 2023;15:917. https://doi.org/10.3390/nu15040917
- Ozer NT, Onuk S, Temel Ş, Yüksel RC, Sungur M, Günoğan K. Association between nutritional Status, energy-protein-micronutrient intake, and mortality in critically ill patients receiving enteral nutrition. J Crit Intensive Care. 2025;16:18-26. https://doi.org/10.14744/dcybd.2025.09450
- Capozzi A, Scambia G, Lello S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas. 2020;140:55-63. https://doi.org/10.1016/j.maturitas.2020.05.020
- Hao G, Zhang B, Gu M, et al. Vitamin K intake and the risk of fractures: a meta-analysis. Medicine (Baltimore). 2017;96:e6725. https://doi.org/10.1097/MD.0000000000006725
- Cashman KD. Vitamin D deficiency: defining, prevalence, causes, and strategies of addressing. Calcif Tissue Int. 2020;106:14-29. https://doi.org/10.1007/s00223-019-00559-4
- Rondanelli M, Faliva MA, Tartara A, et al. An update on magnesium and bone health. Biometals. 2021;34:715-736. https://doi.org/10.1007/s10534-021-00305-0
- Huang Y, Cao D, Chen Z, et al. Iron intake and multiple health outcomes: umbrella review. Crit Rev Food Sci Nutr. 2023;63:2910-2927. https://doi.org/10.1080/10408398.2021.1982861
Copyright and license
Copyright © 2025 The author(s). This is an open-access article under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.










