Abstract
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a rare autosomal recessive metabolic disorder characterized by thymidine phosphorylase deficiency. The progressive course of MNGIE increases the importance of early diagnosis and supportive treatment approaches. The disease usually presents with gastrointestinal dysmotility, neuropathy, hearing loss, cachexia, and nutritional disorders. In this case report, we present the nutritional management and follow-up of a 16-year-old male patient with MNGIE disease. The patient presented with a loss of nearly 40% of his total body weight, a BMI of 9.4 kg/m2 and severe nutritional deficiencies. Total parenteral nutrition was initiated in the first phase of nutritional treatment and then gradually transitioned to enteral nutrition. The patient’s nutritional status was improved with peptide-based formulas and pancreatic enzyme support. During the treatment process, energy and protein requirements were nearly met, weight gain was achieved and biochemical parameters were improved.
Keywords: enteral, malnutrition, mitochondrial neurogastrointestinal encephalopathy syndrome, nutrition, parenteral
Main Points
- Severe gastrointestinal dysmotility and malnutrition are common in patients with MNGIE.
- Nutritional treatment is essential to decrease mortality and improve quality of life in MNGIE disease.
- Severe gastrointestinal dysmotility and malnutrition in MNGIE can be effectively managed with a combination of parenteral and enteral nutrition.
Introduction
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a rare autosomal recessively inherited metabolic disorder caused by thymidine phosphorylase (TP) deficiency due to biallelic pathogenic variants in thymidine phosphorylase (TYMP) gene. Mutations in TYMP gene led to toxic levels of plasma thymidine accumulation, deoxyribonucleoside imbalance, impairment in DNA replication, and eventually mitochondrial disfunction. Approximately 550 cases of MNGIE cases were reported.1,2
Although the disease has a progressive and a fatal course, the quality of life demands basically on nutrition aids and other supportive measurements. Behind the several therapeutic approaches targeting enzyme increment such as TP infusions, hematopoietic stem cell transplantation (HSCT), and orthotopic liver transplantation (OLT), a curative solution is not available. The timing of HSCT and OLT is very critical, as the severity of gastrointestinal (GI) symptoms affects treatment success. Gene therapy is considered as a possible treatment option as it has shown efficacy in preliminary clinical studies.3
Clinically, MNGIE is characterized by neurological involvement manifestations such as ptosis, external ophthalmoplegia, peripheral neuropathy and leukoencephalopathy. Additionally, dramatic gastrointestinal signs and symptoms of dysmotility, abdominal pain, nausea, dysphagia, attacks of pseudoobstruction, and diarrhea led to severe malnutrition and cachexia. Dysphagia both prevents adequate oral intake and also can lead to aspiration and lower respiratory system infections. Different studies put forward that nutritional treatment in pediatric age group provides benefits in terms of clinical course and quality of life. It has been reported that 29-48% of patients with mitochondrial diseases have gastrointestinal involvement and 52% have growth retardation.3-5 Therefore, clinical nutritional treatment is of great importance in MNGIE disease, although there is not a standard approach. Here, we want to share our nutritional management protocol, as it has been succeded in a MNGIE patient.
Case Report
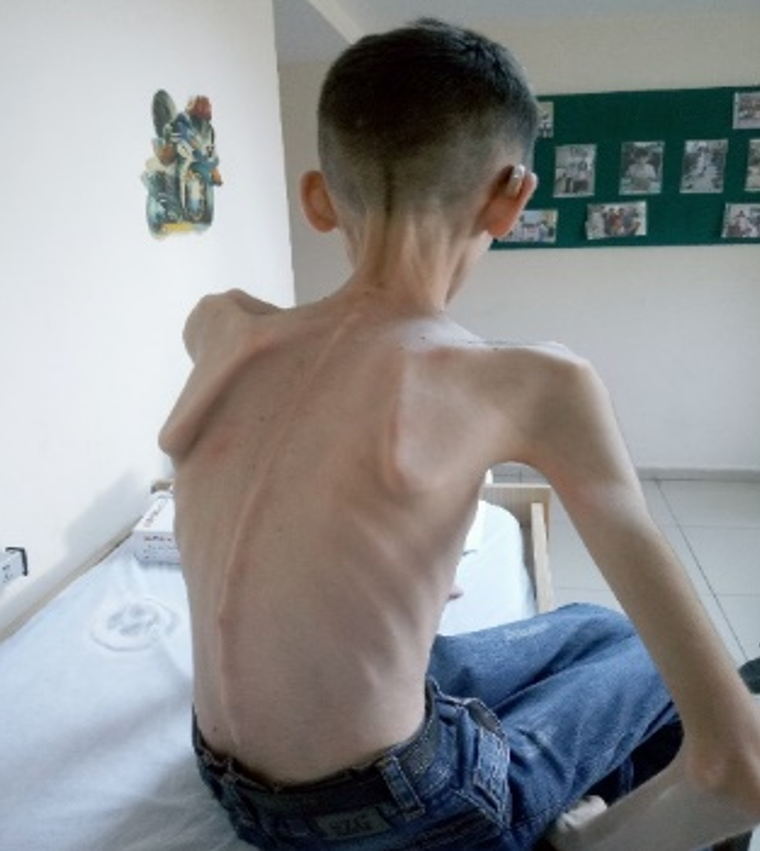
A 16-year-old male patient was admitted to Çukurova University Division of Pediatric Metabolism and Nutrition Department with complaints of GIS dismotility, severe wait loss, and symptoms of malnutrition together with peripheric neuropathy and hearing loss. Family history was unremarkable and there was no consanguinity between the parents. His mental, motor development and physical growth was normal in the first 15 years of life. Only bilateral sensorineural hearing loss was detected at the age of 7 years. Recurrent vomiting attacks, resistant diarrhea and swallowing difficulties due to muscle weakness, which had been began in the last 6 months caused a 15 kg weight loss and severe malnutrition of the patient (Figure 1). He was hospitalized several times with a need of intravenous fluid support. After excluding common causes of these GIS symptoms such as obstructive lessions, inflamatory bowel diseases, and infections concomitant neurological findings led a suspicion of MNGIE disease. A pathogenic variant detected in TYMP gene confirmed the prediagnosis of MNGIE disease.
On admission his weight was 22 kg (-8.09 SDS), height was 153 cm (-3.1 SDS), and body mass index (BMI) was 9.4 kg/m2 (-10.92 SDS). He had bilateral senseronoral hearing loss, and polyneuropaty. He had also difficulty in swallowing solid foods. Initial hematological and biochemical parameters revealed low levels of hemoglobin, hematocrit, total protein, albumin, sodium, potassium, chloride, iron, calcium, phosphorous, and magnesium (Table 1). During the patient’s hospitalization erythrocyte and albumin infusions were given together with vitamin, mineral, and trace elements supplementations.
| Table 1. Laboratory data of the patient. | |||
| Parameter |
|
|
|
| Hemoglobin (g/dL) |
|
|
|
| Hematocrit (%) |
|
|
|
| MCV (fl) |
|
|
|
| BUN (mg/dL) |
|
|
|
| Creatine (mg/dL) |
|
|
|
| Iron (µg/dL) |
|
|
|
| Ferritin (ng/mL) |
|
|
|
| Sodium (mmol/L) |
|
|
|
| Potassium (mmol/L) |
|
|
|
| Chloride (mmol/L) |
|
|
|
| Calcium (mg/dL) |
|
|
|
| Phosphorous (mg/dL) |
|
|
|
| Magnesium (mg/dL) |
|
|
|
| AST (U/L) |
|
|
|
| ALT (U/L) |
|
|
|
| Triglycerides (mg/dL) |
|
|
|
| Total protein (g/dL) |
|
|
|
| Albumin (g/L) |
|
|
|
| B12 (pg/mL) |
|
|
|
| Folate (ng/mL) |
|
|
|
Evaluation of his 24 hours nutritional record with STRONGkids screening tool (5 point), it was found that the patient was in the high-risk group in terms of malnutrition. Then, oral nutrition was stopped and total parenteral nutrition (TPN) was initiated to come over the severe gastric dismotility and secondary malabsorbsition. Our patient was observed to have gastrointestinal symptoms such as diarrhea 5-6 times/day, vomiting and distension 2-3 times/day. The daily energy and protein intake targets were 440-550 kcal (20-25 kcal/kg/day) and 26 g/day (1.2 g/kg actual body weight), respectively. Energy intake was gradually increased to prevent refeeding syndrome (Table 2). On the 10th day of hospitalization, a nasogastric tube was inserted for transition to enteral nutrition and TPN was gradually tapered and terminated 14th day. Pancreatic enzyme supplementation (600U lipase and amylase per 1 gram fat in diet) was started with enteral formula (16x10 ml) containing peptide-based medium-chain triglycerides and glutamine (2x5 g-0,5 g/kg/day) was added to the diet. Enteral formula was gradually increased with an average 10-20% increase in each visit. The patient was discharged on the 27th day of hospitalization with NG removal and 8x50 ml oral nutritional treatment and glutamine in addition to regimen 2 (yoghurt, soup, compote etc.-soft and easily digestible diet) feeding. The patient came for outpatient clinic control on the 15th day of discharge, and his current weight was 24 kg (-7.37 SDS) and his body mass index was 10.25 kg/m2 (-9.23 SDS). He had tolerable gastrointestinal symptoms and signs. Biochemical parameters albumin, total protein, magnesium and calcium levels increased.
Discussion
Due to the rarity and multisystemic involvement of MNGIE, patients have a history of uncountable admissions to different specialties before a specific diagnosis is confirmed. Furthermore, progressive nature of the disease also led to irreversible complications and a poor prognosis.2
Severe weight loss and cachexia related to gastrointestinal dysmotility is one of the main problems that are observed in MNGIE. Therefore, nutritional treatment is essential to decrease mortality and improve quality of life in these patients. Nutritional treatment in patients with MNGIE aims to increase nutrient intake with enteral/parenteral nutrition. Feeding tubes are used in cases where necessary. Nutritional treatment has beneficial effects on both decreasing the severity of malnutrition and the other complications. TPN together with enteral nutrition generally prevents refractory cachexia and helps to achieve some weight gain. Although metabolic problems such as hypokalemia, elevated liver function tests and sepsis are the probable side effects associated with TPN, both in the long or short term of MINGIE management TPN is still a gold standard in nutritional treatment.1 In patients with limited oral intake due to gastrointestinal symptoms, enteral tube feeding may improve quality of life and growth failure. In our patient, reduction in gastrointestinal symptoms and recurrent vomiting attacks, weight gain and improvement in quality of life were observed after tube feeding treatment. There are very limited numbers of case reports about the nutritional management of MNGIE patients. It was notified that a 23-year-old female MNGIE patient with a BMI of 15 kg/m2 was treated with a hypercaloric diet containing 55% carbohydrate, 16% protein and 29% fat. Initially the patient was unable to finish this diet due to early feeling of satiety and anorexia, however she gradually reached the recommended energy intake. At the end of the first year of follow-up with this diat, the patient’s BMI was increased to 16.9 kg/m2 and muscle strength which was measured by hand grip improved. Also, a significant improvement in biochemical parameters was observed.6 Another hopeful report of a 36-year-old severely malnutreted MNGIE patient who had recurrent diarrhea, the initial BMI of 11.9 kg/m2 was improved to 12.7 kg/m2 in the first year of total parenteral nutrition. On the last visit the patient was 43 years old and has been receiving home nutrition therapy with an acceptable quality of life for more than 6 years.7 Similar to the cases reported in the literature, the body mass index of our patient increased from 9.4 kg/m2 (-10.92 SDS) to 10.25 kg/m2 (-9.23 SDS) with nutritional treatment. So, until a curative treatment option is available, one of the most important goals should be to alleviate the symptoms and increase the resilience of MNGIE patients, thereby improving their quality of life, even if only slightly, and ensuring survival.
Limitations
The fact that nutritional laboratory parameters such as prealbumin and transferrin were not examined and that anthropometric measurements or body composition other than body weight were not taken were counted as limitations.
Conclusion
Nutritional treatment has a critical role in MNGIE disease management. The combined usage of parenteral and enteral nutrition is a relatively effective treatment approach, especially in cases of severe gastrointestinal dysmotility and malnutrition. The case that we reported demonstrated the positive effect of diet on quality of life and somehow in prognosis.
Ethical approval
Written consent was obtained from the patient’s parents.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Kasti A, Nikolaki M, Pyrousis I, Synodinou K, Oikonomopoulos N, Triantafyllou K. Intensive nutrition support may benefit patients with a rare mitochondrial disorder. Nutr Clin Pract. 2022;37:361-365. https://doi.org/10.1002/ncp.10726
- Redha N, Al-Sahlawi Z, Hasan H, Ghareeb S, Humaidan H. Meningoencephalitis in a novel mutation in MNGIE (mitochondrial neurogastrointestinal encephalomyopathy) ending a familial diagnostic odyssey: A case series report. J Cent Nerv Syst Dis. 2024;16:11795735241241423. https://doi.org/10.1177/11795735241241423
- Hirano M, Carelli V, De Giorgio R, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Position paper on diagnosis, prognosis, and treatment by the MNGIE International Network. J Inherit Metab Dis. 2021;44:376-387. https://doi.org/10.1002/jimd.12300
- Wang J, Chen W, Wang F, et al. Nutrition Therapy for Mitochondrial Neurogastrointestinal Encephalopathy with Homozygous Mutation of the TYMP Gene. Clin Nutr Res. 2015;4:132-136. https://doi.org/10.7762/cnr.2015.4.2.132
- Choi HS, Lee YM. Enteral Tube Feeding in Paediatric Mitochondrial Diseases. Sci Rep. 2017;7:16909. https://doi.org/10.1038/s41598-017-17256-7
- Paolini B, Ciondolo ID, Lapini E, et al. Nutritional treatment in a patient affected by Mithochondrial Neuro-Gastrointestinal Encephalomyopathy (MNGIE). Med J Nutrition Metab. 2014;7:173-179. https://doi.org/10.3233/MNM-140017
- Barisic A, Ljubas Kelecic D, Vranesic Bender D, et al. Case report: A patient with mitochondrial neurogastrointestinal encephalomyopathy and chronic intestinal failure. Front Nutr. 2022;9:983873. https://doi.org/10.3389/fnut.2022.983873
Copyright and license
Copyright © 2025 The author(s). This is an open-access article under the terms of the Creative Commons Attribution License (CC BY) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.